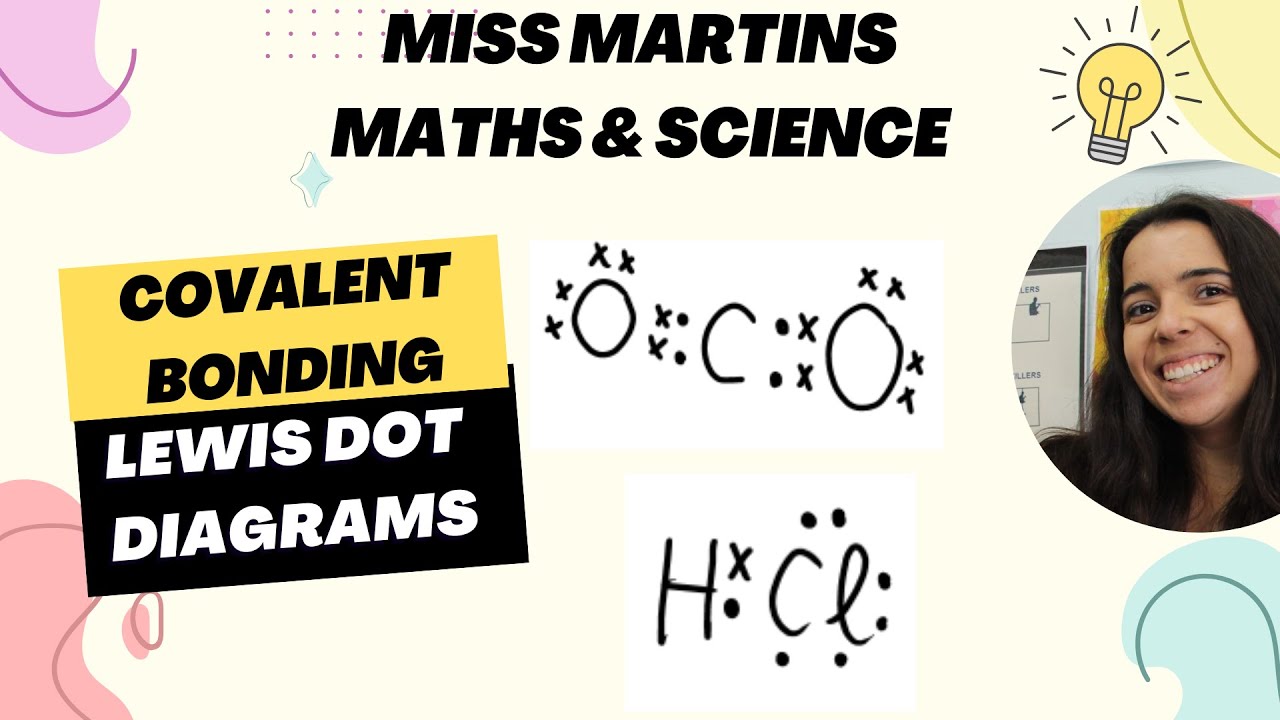Chemical Bonds: Ionic and Covalent
Summary
TLDRThis video explains how atoms, much like people, prefer bonding to achieve stability. Atoms bond by losing, gaining, or sharing electrons to fill their valence shells, adhering to the octet rule. Ionic bonds form when electrons transfer between metals and non-metals, creating oppositely charged ions, as seen in sodium chloride. Covalent bonds, on the other hand, involve sharing electrons, as in H2 and HCl molecules. These bonds create the vast array of compounds essential to life. The video highlights the diversity of compounds formed from 90 naturally occurring elements, vital to life on Earth and beyond.
Takeaways
- 🔬 Atoms, like people, can be loners but often prefer to bond to achieve stability.
- ⚛️ Atoms aim to reach the most stable, lowest energy state by filling their outer valence shell with electrons.
- 🧪 The octet rule states that many atoms need eight electrons to fill their valence shell, but hydrogen and helium are exceptions, needing only two.
- 🔋 Ionic bonds occur when electrons are transferred from a metal to a non-metal, creating oppositely charged ions that attract each other.
- 🧲 Sodium chloride (table salt) is an example of an ionic compound, where sodium loses an electron and becomes a cation, and chlorine gains that electron, becoming an anion.
- 💎 Ionic compounds don't exist as individual units but form repeating structures called crystal lattices, like the beams in Mexico's Cave of Crystals.
- 🧬 Covalent bonds form when atoms share electrons, creating a new orbital that extends around both atoms' nuclei, producing a molecule.
- ⚖️ Nonpolar covalent bonds occur when electrons are shared equally between atoms, while polar covalent bonds result from unequal sharing of electrons.
- 🌍 Atoms can form multiple covalent bonds, allowing for the creation of complex molecules, such as caffeine.
- 🔭 The variety of compounds formed from the relationships between atoms is essential to life on Earth and could potentially exist beyond our planet.
Q & A
Why do most atoms prefer to bond rather than remain unbonded?
-Atoms prefer to bond because they seek to reach their most stable, lowest energy state, which often occurs when their outermost electron shell, or valence shell, is filled.
What is the significance of an atom's valence shell?
-An atom's valence shell is important because it determines the atom's stability. Atoms are most stable when this outermost shell is completely filled with electrons, often following the octet rule.
What is the octet rule and are there exceptions to it?
-The octet rule states that atoms are stable when their valence shell contains eight electrons. However, hydrogen and helium are exceptions, needing only two electrons to fill their valence shell.
How do atoms achieve a full valence shell?
-Atoms achieve a full valence shell by losing, gaining, or sharing valence electrons through chemical bonds.
What happens in an ionic bond?
-In an ionic bond, electrons are transferred from a metal to a non-metal, creating oppositely charged ions that are held together by electrostatic attraction.
Can you give an example of an ionic compound and explain how it forms?
-Table salt (sodium chloride) is an example of an ionic compound. Sodium (a metal) transfers its one valence electron to chlorine (a non-metal), resulting in the formation of a positively charged sodium ion (cation) and a negatively charged chloride ion (anion), which then bond together.
What is the difference between ionic and covalent bonds?
-Ionic bonds involve the transfer of electrons from one atom to another, typically between a metal and a non-metal, while covalent bonds involve the sharing of electrons between atoms, usually between non-metals.
What is a nonpolar covalent bond?
-A nonpolar covalent bond occurs when electrons are shared evenly between atoms, resulting in no partial charges on either atom.
What happens in a polar covalent bond?
-In a polar covalent bond, electrons are shared unequally between atoms, causing one atom to carry a partial negative charge and the other a partial positive charge.
How does the number of bonds an atom can form vary between elements?
-Different elements can form different numbers of covalent bonds. For example, hydrogen can form 1 bond, oxygen 2, nitrogen 3, and carbon 4.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahora5.0 / 5 (0 votes)