Dalton's Law
Summary
TLDRThis educational video script outlines a chemistry experiment to demonstrate Dalton's Law of Partial Pressures. Key materials include balloons, bottles, gloves, masks, lab gowns, vinegar, baking soda, hydrogen peroxide, and bleach. The experiment involves mixing vinegar with baking soda to produce carbon dioxide and hydrogen peroxide with bleach to generate oxygen. The resulting gas pressures are visually represented by the inflation of balloons, illustrating the sum of individual gas pressures.
Takeaways
- 🧪 The experiment involves mixing vinegar and baking soda to create carbon dioxide gas.
- 🧑🔬 The purpose is to understand Dalton's Law of Partial Pressures and to measure individual gas pressures.
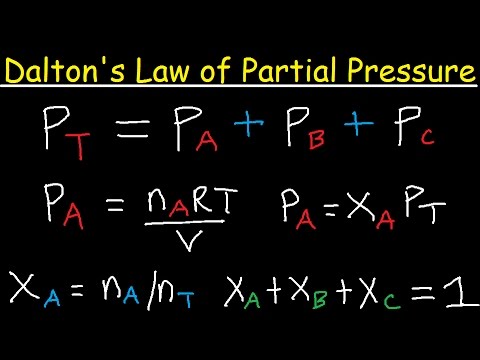
- 🌟 Dalton's Law states that the total pressure of a gas mixture is the sum of the partial pressures of each individual gas.
- 🧴 Safety equipment such as gloves, masks, and lab gowns are used to protect from chemical exposure.
- 🎈 A balloon is filled with baking soda and placed on a bottle containing vinegar to observe the gas production.
- 🔬 The experiment also uses hydrogen peroxide and bleach to produce oxygen gas, requiring the bleach to contain sodium hypochlorite.
- 📈 The size of the balloon indicates the amount of pressure produced by the gas.
- 🔍 The experiment demonstrates that the total pressure is the sum of the partial pressures of different gas mixtures.
- 📊 The final balloon is larger in the last experiment, showing the combined effect of oxygen and carbon dioxide pressures.
- 🏷️ The script describes a series of experiments to visually demonstrate the principles of gas laws.
Q & A
What are the main materials used in the first part of the experiment?
-The main materials used in the first part of the experiment are a balloon, an empty bottle, a funnel, gloves, a face mask, a lab gown, vinegar, and baking soda.
What is the objective of the first experiment?
-The objectives of the first experiment are to understand Dalton's law of partial pressures, to determine the individual pressure of each gas, and to prove that the total pressure of a gas is the sum of the partial pressures of each individual gas.
How is the balloon filled with baking soda in the experiment?
-The balloon is filled with baking soda by using a funnel to pour the baking soda into the balloon.
What happens when the balloon filled with baking soda is placed on top of the vinegar-filled bottle?
-When the balloon filled with baking soda is placed on top of the vinegar-filled bottle, the two react to produce carbon dioxide gas, which causes the balloon to expand.
What is the purpose of gloves, face masks, and lab gowns in the experiment?
-Gloves, face masks, and lab gowns are used in the experiment to protect the experimenter from unnecessary exposure to chemicals.
What is Dalton's law of partial pressures?
-Dalton's law of partial pressures states that the total pressure exerted by a mixture of non-reactive gases is equal to the sum of the partial pressures of the individual gases.
What is the role of vinegar in the experiment?
-Vinegar plays a role in the experiment by reacting with baking soda to produce carbon dioxide gas, which is used to demonstrate the concept of partial pressures.
How is the hydrogen peroxide mixed with bleach in the second part of the experiment?
-In the second part of the experiment, the hydrogen peroxide is mixed with bleach by pouring it into a bottle filled with bleach and then shaking the bottle to mix the chemicals.
What gas is produced when hydrogen peroxide and bleach are mixed together?
-When hydrogen peroxide and bleach are mixed together, they produce oxygen gas.
What is the significance of the balloon expanding in the experiment?
-The expansion of the balloon signifies the production of gas and an increase in pressure within the bottle. The size of the balloon indicates the amount of pressure produced by the chemical reaction.
How does the experiment demonstrate the total pressure of different gas mixtures?
-The experiment demonstrates the total pressure of different gas mixtures by showing that the final balloon size, which is the result of mixing oxygen and carbon dioxide, is larger than the balloons from the individual reactions, indicating the sum of the partial pressures.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados
5.0 / 5 (0 votes)