Physical and Chemical Properties and Changes
Summary
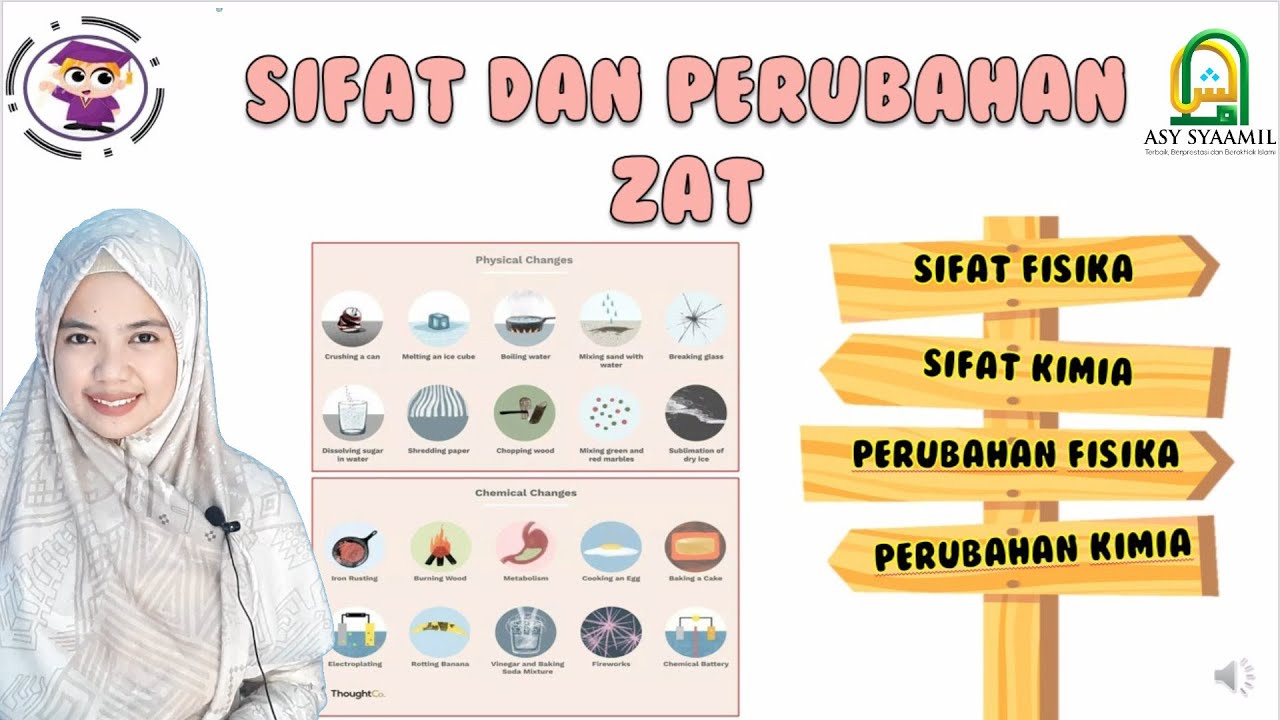
TLDRThis lesson covers the differences between physical and chemical properties and changes. Physical properties describe observable traits like color, texture, and mass, while chemical properties describe how substances react, such as flammability or oxidation. The instructor uses relatable examples, comparing chemical properties to personality traits and reactions to social situations. Physical changes involve altering appearance without forming new substances (like boiling water), while chemical changes result in new substances (like burning paper). Key concepts include precipitate formation, gas release, and temperature changes.
Takeaways
- 😀 Physical properties describe the appearance, smell, feel, and measurable characteristics of a substance.
- 👀 Examples of physical properties include color, texture, size, volume, mass, density, buoyancy, boiling point, and melting point.
- 🌡️ Chemical properties describe how a substance reacts or does not react, similar to how personality reflects behavior.
- 💥 Common chemical properties include oxidation, rusting, flammability, and reactions with other substances.
- 🧪 Physical changes involve changes in appearance or state without forming a new substance, like boiling, freezing, or crushing.
- 🔥 Chemical changes result in a new substance due to bond-breaking and rearranging of atoms, such as combustion or rusting.
- 🍎 Indicators of chemical reactions include color changes, gas formation, precipitate formation, new smells, and temperature changes.
- 📏 All phase changes, like melting, freezing, and boiling, are considered physical changes because the substance remains the same.
- ⚗️ Chemical reactions can be indicated by unexpected temperature changes or the generation of light, such as in fire or glow sticks.
- 📚 Physical and chemical properties, along with their changes, are fundamental concepts in chemistry, often seen as vocabulary.
Q & A
What is a physical property?
-A physical property describes the way something looks, feels, or smells, and can sometimes be measured. Examples include color, texture, size, mass, and volume.
What is an example of a physical property?
-Examples of physical properties include the rough texture of a pine cone, the brownish-red color of someone's hair, or the boiling point of water.
What distinguishes a chemical property from a physical property?
-A chemical property describes how a substance reacts or does not react, which is often based on the number of valence electrons, while a physical property describes observable characteristics like size, shape, or texture.
How does the script relate chemical properties to personality traits?
-The script compares chemical properties to personality traits, suggesting that just as chemical properties describe how substances react, personality traits describe how a person reacts in certain situations.
What is a physical change?
-A physical change is when something changes in appearance but no new substance is formed. For example, crushing a can or freezing water are physical changes.
Can you give examples of physical changes?
-Yes, examples include melting and freezing water, boiling water, breaking an object, or crushing a crystal into smaller pieces.
What is a chemical change?
-A chemical change occurs when a new substance is formed due to the breaking and rearranging of chemical bonds. Examples include rusting, burning paper, and the formation of a precipitate.
What are some signs that a chemical reaction has occurred?
-Signs of a chemical reaction include a color change, the formation of gas, a new smell, temperature change, or the generation of light, such as when a glow stick glows.
How is the formation of a precipitate described in the script?
-The formation of a precipitate occurs when two solutions react to form a solid. An example would be mixing vinegar and baking soda, which produces gas and sometimes a solid precipitate.
What are examples of chemical properties and changes mentioned in the script?
-Chemical properties mentioned include flammability and oxidation (like an apple turning brown). Chemical changes include burning paper and the reaction of vinegar with baking soda.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados

General Chemistry 1 - Matter and Its Properties

Grade 9 Chemistry, Lesson 3 - Physical and Chemical Properties and Changes

Physical and Chemical Properties of Matter
SIFAT DAN PERUBAHAN ZAT | SIFAT FISIKA DAN KIMIA | PERUBAHAN FISIKA DAN KIMIA

Zat dan Perubahannya

Is Matter around us Pure? Full Chapter (Animation) | Class 9 Science chapter 2 | CBSE | NCERT
5.0 / 5 (0 votes)
