What Is The Bronsted Lowry Theory | Acids, Bases & Alkali's | Chemistry | FuseSchool
Summary
TLDRLa teoría de ácidos y bases de Bronsted-Lowry amplía la definición de Arrhenius, destacando que un ácido es una sustancia que dona iones H+ y una base es una que los acepta. A diferencia de Arrhenius, donde las bases deben liberar OH-, según Bronsted-Lowry, una base puede aceptar H+ sin necesidad de tener un pH mayor a 7. El agua puede actuar como ácido o base dependiendo de la situación, siendo un ejemplo de sustancia anfotérica. El proceso de reacción ácido-base en esta teoría se basa en la transferencia de protones (H+), sin importar la pH del compuesto.
Takeaways
- 😀 La teoría de Arrhenius define a un ácido como una sustancia que libera iones H+ en solución, y a una base como una sustancia que libera iones OH- en solución.
- 😀 El amoníaco (NH3) actúa como una base, aunque no contiene iones OH-, lo que plantea la necesidad de una teoría más amplia.
- 😀 En la teoría de Bronsted-Lowry, un ácido es una sustancia que dona iones H+, mientras que una base es una sustancia que acepta iones H+.
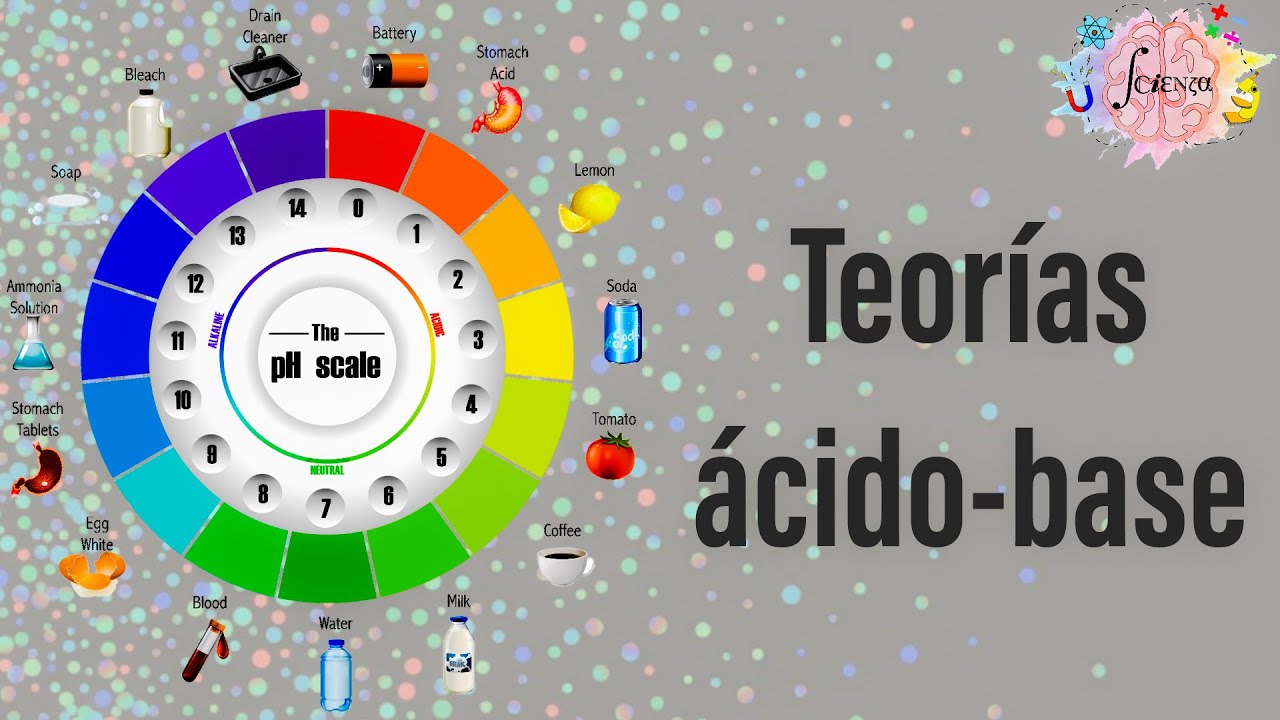
- 😀 Los ácidos tienen un pH menor a 7, y se pueden identificar mediante el indicador universal o papel indicador, que cambia de color (rojo o naranja) según la fuerza del ácido.
- 😀 Un ejemplo común de ácido es el ácido clorhídrico (HCl), que se disocia en agua para formar H+ y Cl-.
- 😀 La reacción de HCl con agua forma iones hidronio (H3O+), lo que muestra cómo un ácido transfiere iones H+ a otra sustancia.
- 😀 La teoría de Bronsted-Lowry amplía la definición de ácidos y bases al enfocarse en la transferencia de iones H+, no en el pH.
- 😀 El amoníaco (NH3) al disolverse en agua acepta un ion H+ y forma iones amonio (NH4+) y OH-, actuando como base en esta reacción.
- 😀 En la reacción entre H2O y NH3, el agua actúa como un ácido (libera H+), mientras que el amoníaco actúa como una base (acepta H+).
- 😀 El agua puede actuar como un ácido o una base, dependiendo de la sustancia con la que reaccione. Esto la convierte en una sustancia anfótera, capaz de actuar como ácido y base.
- 😀 Según la teoría de Bronsted-Lowry, una reacción ácido-base siempre involucra la transferencia de un protón (H+).
Q & A
¿Qué define la teoría de Arrhenius sobre los ácidos y bases?
-La teoría de Arrhenius define a un ácido como una sustancia que libera iones H+ en solución, y una base como una sustancia que libera iones OH- en solución.
¿Cómo puede el amoníaco (NH3) actuar como base si no contiene iones OH-?
-El amoníaco actúa como base según la teoría de Bronsted-Lowry al aceptar un ion H+ del agua, formando iones NH4+ y OH-, lo que no requiere la presencia de iones OH- en el amoníaco.
¿Qué propiedad de los ácidos se puede identificar usando un indicador universal?
-Los ácidos se pueden identificar porque el indicador universal cambia de color, pasando de verde a rojo o naranja dependiendo de la fuerza del ácido.
¿Qué ocurre cuando el cloruro de hidrógeno (HCl) se disuelve en agua?
-Cuando el cloruro de hidrógeno se disuelve en agua, se disocia para formar un ion H+ y un ion Cl-, y el H+ es transferido al agua, formando un ion hidronio (H3O+).
Según la teoría de Bronsted-Lowry, ¿qué es un ácido?
-Según la teoría de Bronsted-Lowry, un ácido es una sustancia que dona un ion H+ en solución.
¿Qué es un base según la teoría de Bronsted-Lowry?
-Un base, según la teoría de Bronsted-Lowry, es una sustancia que acepta un ion H+ en solución.
¿Qué sustancia actúa como ácido en el ejemplo de amoníaco (NH3) y agua?
-En este ejemplo, el agua actúa como ácido porque dona un ion H+ al amoníaco.
¿Qué significa que una sustancia sea anfótera?
-Una sustancia es anfótera si puede actuar tanto como ácido como base, dependiendo de las condiciones. El agua, por ejemplo, puede actuar como ácido en un caso y como base en otro.
¿Cuál es la diferencia entre las definiciones de ácido en la teoría de Arrhenius y la de Bronsted-Lowry?
-En la teoría de Arrhenius, un ácido es una sustancia que libera iones H+ en solución, mientras que en la teoría de Bronsted-Lowry, un ácido es una sustancia que dona un ion H+ a otra sustancia.
¿Qué ocurre durante una reacción ácido-base según la teoría de Bronsted-Lowry?
-Según la teoría de Bronsted-Lowry, una reacción ácido-base involucra la transferencia de un ion H+ de una sustancia a otra.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados

14-Equilibrios Ácido-Base. 1.0 Definiciones de Arrhenius, Bronsted-Lowry y Lewis.
Teorías ácido - base (Arrhenius, Bronsted-Lowry, Lewis)

Teroía Brönsted y Lowry ácido-base

Teoría de Arrhenius ácido-base

Teoría de Lewis ácido-base

⚠️Teorías Ácido-Base | Arrhenius, Bronsted-Lowry, Lewis⚠️ [Fácil y Rápido] | QUÍMICA |

Definiciones de ácido-base
5.0 / 5 (0 votes)