Tekanan Uap Larutan Non Elektrolit | Kimia SMA | Tetty Afianti
Summary
TLDRIn this chemistry lesson, the topic of vapor pressure in non-electrolyte solutions is explored, focusing on formulas and calculations for determining vapor pressure changes. The lesson covers the basics of non-electrolyte solutions, such as urea, glucose, and glycerol, and demonstrates how to use mole fractions and solvent-solute moles in calculations. Several example problems are worked through, including glycerol-water, glucose-water, and sucrose-water solutions, explaining how to calculate vapor pressure decrease and final vapor pressures. The lesson concludes with exercises on relative molecular mass determination, providing viewers with practical insights into the subject.
Takeaways
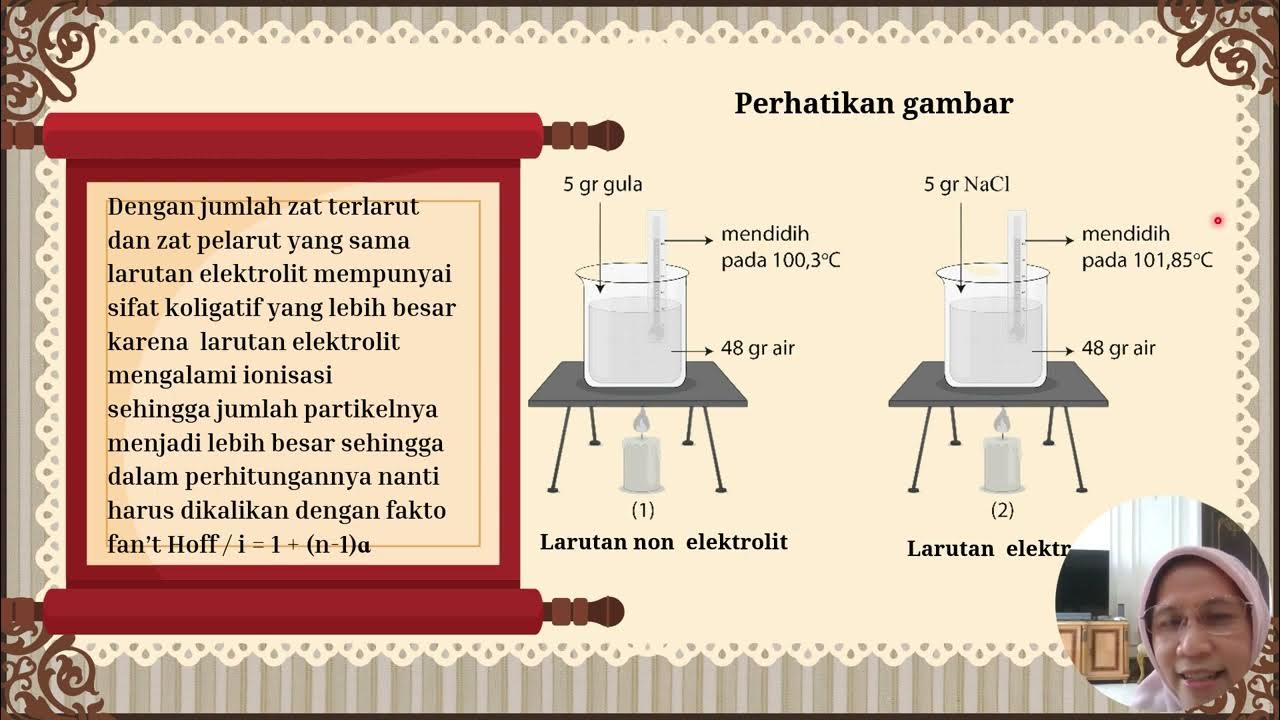
- 😀 Non-electrolyte solutions cannot conduct electricity and include substances like urea, glucose, sucrose, and glycerol.
- 😀 The vapor pressure of non-electrolyte solutions can be determined using the formula: P = P₀ × Xₚ (where P₀ is the vapor pressure of the pure solvent, and Xₚ is the mole fraction of the solvent).
- 😀 The decrease in vapor pressure (ΔP) can be calculated using the formula: ΔP = P₀ × Xₜ (where Xₜ is the mole fraction of the solute).
- 😀 The formula for calculating ΔP can also be represented as ΔP = P₀ × (moles of solute / (moles of solute + moles of solvent)).
- 😀 The mole fraction (X) is calculated by dividing the moles of a component by the total moles in the solution.
- 😀 In example 1, when glycerol is dissolved in water, the decrease in vapor pressure is calculated to be 7.5 mm Hg, and the vapor pressure of the solution is 22.5 mm Hg.
- 😀 In example 2, the decrease in vapor pressure of an 18% glucose solution is 0.59 cm Hg, with a vapor pressure of the solution being 27.40 cm Hg.
- 😀 In example 3, a sucrose solution results in a vapor pressure of 16.8 atm when dissolved in water, based on the given molecular weights and the vapor pressure of pure water.
- 😀 In example 4, the molar mass of mannitol is determined to be 180 g/mol after calculating the decrease in vapor pressure in a mannitol-water solution.
- 😀 In example 5, the molecular weight of substance X is found to be 62 g/mol by calculating the decrease in vapor pressure of a solution containing X dissolved in water.
Q & A
What is a non-electrolyte solution?
-A non-electrolyte solution is a solution that cannot conduct electricity. Examples of non-electrolyte solutions include urea, glucose, sucrose, and glycerol.
What formula is used to calculate the vapor pressure of non-electrolyte solutions?
-The formula used is p = p0 * Xp, where p0 is the vapor pressure of the pure solvent and Xp is the mole fraction of the solvent.
What does the delta P formula calculate?
-The delta P formula calculates the decrease in the vapor pressure of the solution. It is given by ΔP = p0 * Xs, where Xs is the mole fraction of the solute.
What is the relationship between the vapor pressure of the solution and the vapor pressure of the pure solvent?
-The vapor pressure of the solution (p) is lower than that of the pure solvent (p0) due to the presence of the solute. The decrease in vapor pressure is calculated using ΔP = p0 * Xs.
How is the decrease in vapor pressure calculated in the example with glycerol and water?
-The decrease in vapor pressure is calculated by using the formula ΔP = p0 * (moles of solute / (moles of solvent + moles of solute)). For glycerol in water, the result was 7.5 mm Hg.
What is the formula to calculate the vapor pressure of a solution after determining the decrease in vapor pressure?
-The vapor pressure of the solution is calculated using the formula p = p0 - ΔP, where p0 is the vapor pressure of the pure solvent and ΔP is the decrease in vapor pressure.
In the second example, how is the decrease in vapor pressure of the glucose solution determined?
-The decrease in vapor pressure for the glucose solution is calculated using the formula ΔP = p0 * (moles of solute / (moles of solvent + moles of solute)), which resulted in a decrease of 0.59 cm Hg.
How do you calculate the vapor pressure of the glucose solution in the second example?
-After calculating the decrease in vapor pressure (0.59 cm Hg), the vapor pressure of the solution is found using the formula p = p0 - ΔP, which gave a result of 27.40 cm Hg.
What is the procedure for calculating the vapor pressure of a sucrose solution in the third example?
-To calculate the vapor pressure of the sucrose solution, you use the formula p = p0 * (moles of solvent / (moles of solvent + moles of solute)). The final result was 16.8 atm.
How is the molecular mass of mannitol determined in the fourth example?
-The molecular mass of mannitol is determined by first calculating the moles of solute, then using the formula Mr = G / moles, where G is the mass of the solute. The result was 184 g/mol.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

SKL (1) | Penurunan Tekanan Uap (∆P) | Kimia Kelas 12
Sifat Koligatif 2Penurunan Tekanan Uap

SIFAT KOLIGATIF LARUTAN : PENURUNAN TEKANAN UAP

3 1 1 Penurunan Tekanan Uap, XII MIPA

Sifat Koligatif Larutan -Kimia SMA kelas 12 semester 1

Simplest Way To Understand Boiling Point Elevation & Vapor Pressure Depression
5.0 / 5 (0 votes)
