Konsep tentang foton - fisika kuantum kelas 12
Summary
TLDRThis educational video delves into the concept of wave-particle duality, focusing on photons. It introduces the discovery and definition of photons, explaining that they are quantum packets of energy in electromagnetic radiation. The script covers the unique properties of photons, such as their lack of mass, charge, and their ability to travel at the speed of light. It also highlights the dual behavior of photons, acting as both waves and particles, and explores the mathematical formula for photon energy. Additionally, the video discusses the unit of energy, electron volts, and their conversion to Joules, providing a comprehensive overview of the topic.
Takeaways
- 😀 Foton is a quantum of energy or a packet of light energy in the form of electromagnetic radiation.
- 😀 Foton is a fundamental particle that forms the basic unit of electromagnetic radiation, including radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays.
- 😀 Foton has no mass and no charge, and is a stable particle moving at the speed of light (3 x 10^8 m/s).
- 😀 Foton can interact with other particles, such as electrons, and one of the phenomena to study is the Compton effect.
- 😀 The concept of wave-particle duality is important for photons, meaning they can behave both as waves and particles.
- 😀 Foton can act as a wave, exhibiting properties like reflection and refraction.
- 😀 Foton can act as a particle, possessing momentum.
- 😀 The energy of a photon is directly proportional to its frequency and is mathematically expressed as E = h * f, where 'h' is Planck's constant.
- 😀 The energy of a photon can also be expressed in terms of its wavelength as E = hc / λ, where λ is the wavelength and c is the speed of light.
- 😀 The energy for a group of photons is the sum of individual photon energies, calculated as E = NH * c / λ.
- 😀 Photon energy can also be measured in electron volts (eV), where 1 eV is the energy gained by an electron when accelerated by a 1-volt potential difference in a vacuum, and 1 eV = 1.6 x 10^-19 J.
Q & A
What is the main topic of the lesson in the script?
-The main topic of the lesson is the concept of dualism of wave-particle, focusing on the photon, its properties, and energy.
Who discovered the concept of the photon, and what is his background?
-The photon concept was discovered by a German theoretical physicist, Max Planck.
What is a photon?
-A photon is a quantum of energy or a packet of light energy in the form of electromagnetic radiation. It can be emitted or absorbed by matter.
What types of electromagnetic waves are considered part of the photon spectrum?
-The photon spectrum includes radio waves, microwaves, infrared light, visible light, ultraviolet light, X-rays, and gamma rays.
What are the properties of a photon?
-Photons have no mass, no charge, they are stable particles, and they travel at the speed of light (3 x 10^8 m/s).
Can a photon interact with other particles?
-Yes, a photon can interact with other particles, such as electrons, which is explored through the Compton effect.
What is meant by 'wave-particle duality' of photons?
-Wave-particle duality refers to the ability of photons to behave both as waves (showing wave-like properties such as reflection and refraction) and as particles (exhibiting momentum).
How is the energy of a photon determined?
-The energy of a photon is directly proportional to its frequency. The formula for photon energy is E = h * f, where h is Planck's constant and f is the frequency.
What is the relationship between photon energy and wavelength?
-The energy of a photon is inversely proportional to its wavelength. This relationship is represented by the equation E = hc / λ, where c is the speed of light and λ is the wavelength.
What is the unit of photon energy commonly used in nuclear and particle physics?
-The unit of photon energy commonly used in nuclear and particle physics is the electron volt (eV). One electron volt is equal to the energy gained or lost by an electron when it moves through a potential difference of 1 volt.
How do you convert electron volts (eV) to joules (J)?
-1 electron volt (eV) is equal to 1.6 x 10^-19 joules (J).
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen
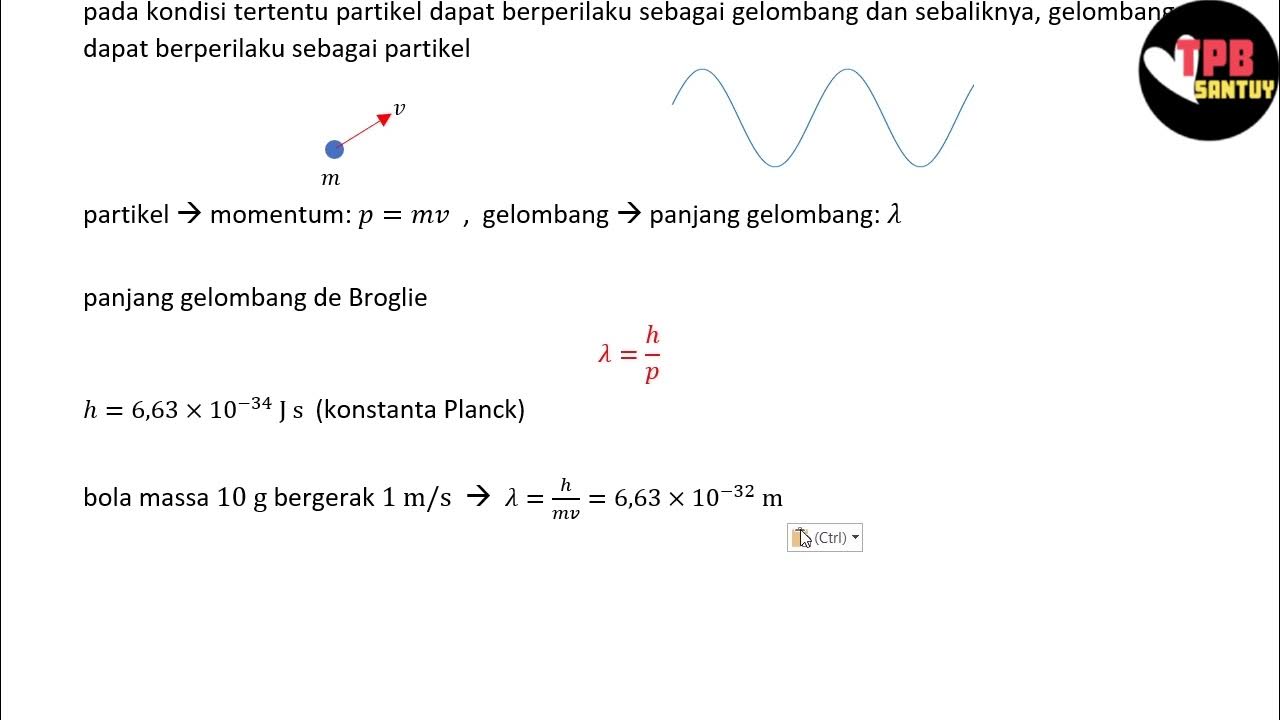
Dualisme Gelombang-Partikel | Fenomena Kuantum | Part 1 | Fisika Dasar
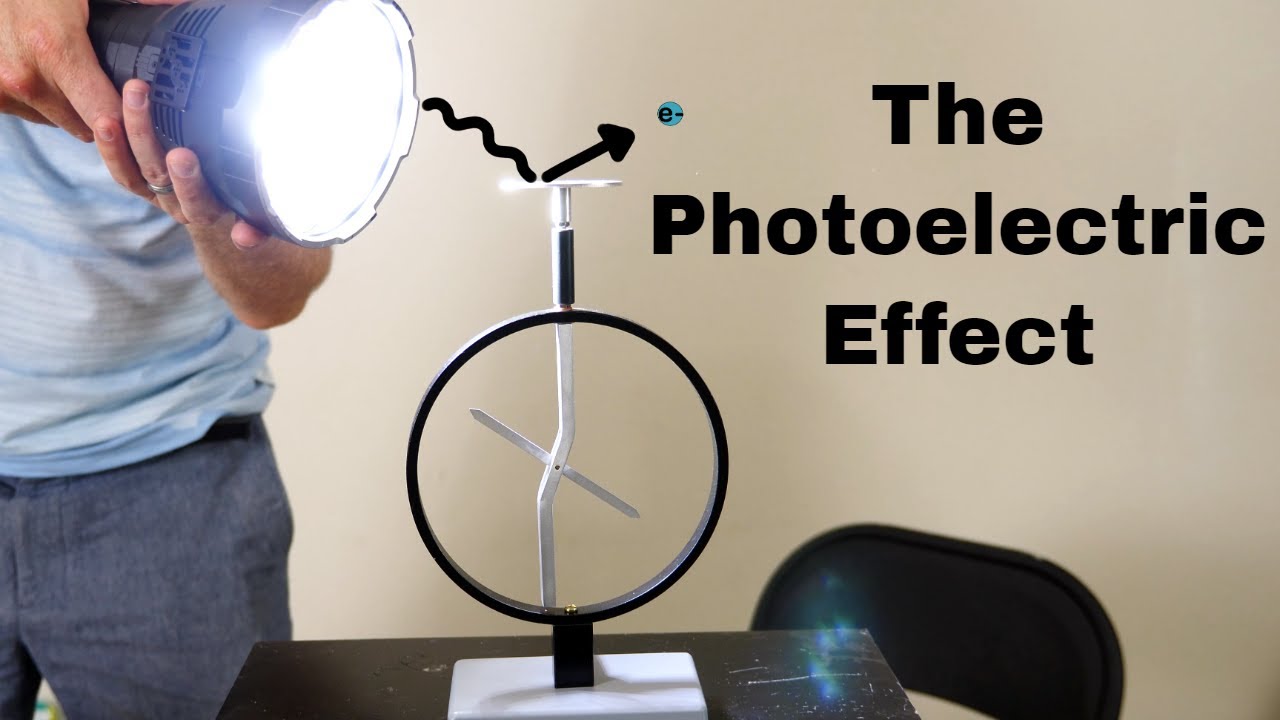
Knocking Electrons With Light—The Photoelectric Effect

Top 6 Discoveries By Albert Einstein || The Great Theories By Einstein || Explained ||

The Weird Experiment that Changes When Observed

The Double-Slit Experiment
Wave-Particle Duality and the Photoelectric Effect
5.0 / 5 (0 votes)
