Wave-Particle Duality and the Photoelectric Effect
Summary
TLDRIn this video, Professor Dave explains the concept of wave-particle duality using light as a key example. He outlines how light was once understood as an electromagnetic wave but couldn't explain phenomena like the photoelectric effect. This led to Einstein's groundbreaking idea that light consists of particles called photons, each with energy determined by frequency. The video delves into quantum theory, revealing how everything, including energy and light, is quantized. Professor Dave highlights the pivotal shift in physics brought about by these discoveries, changing our understanding of the universe and ushering in the quantum revolution.
Takeaways
- 😀 Light behaves as an electromagnetic wave and is also known as electromagnetic radiation.
- 😀 Light waves have two key properties: wavelength (distance from tip to tip) and frequency (number of waves passing a point per unit time).
- 😀 Wavelength and frequency are inversely proportional: shorter wavelengths correspond to higher frequencies.
- 😀 The relationship between wavelength, frequency, and the speed of light is described by a key equation where 'c' is the speed of light (300 million meters per second).
- 😀 The electromagnetic spectrum includes all wavelengths of light, from gamma rays to radio waves, with visible light being the small section we can detect with our eyes.
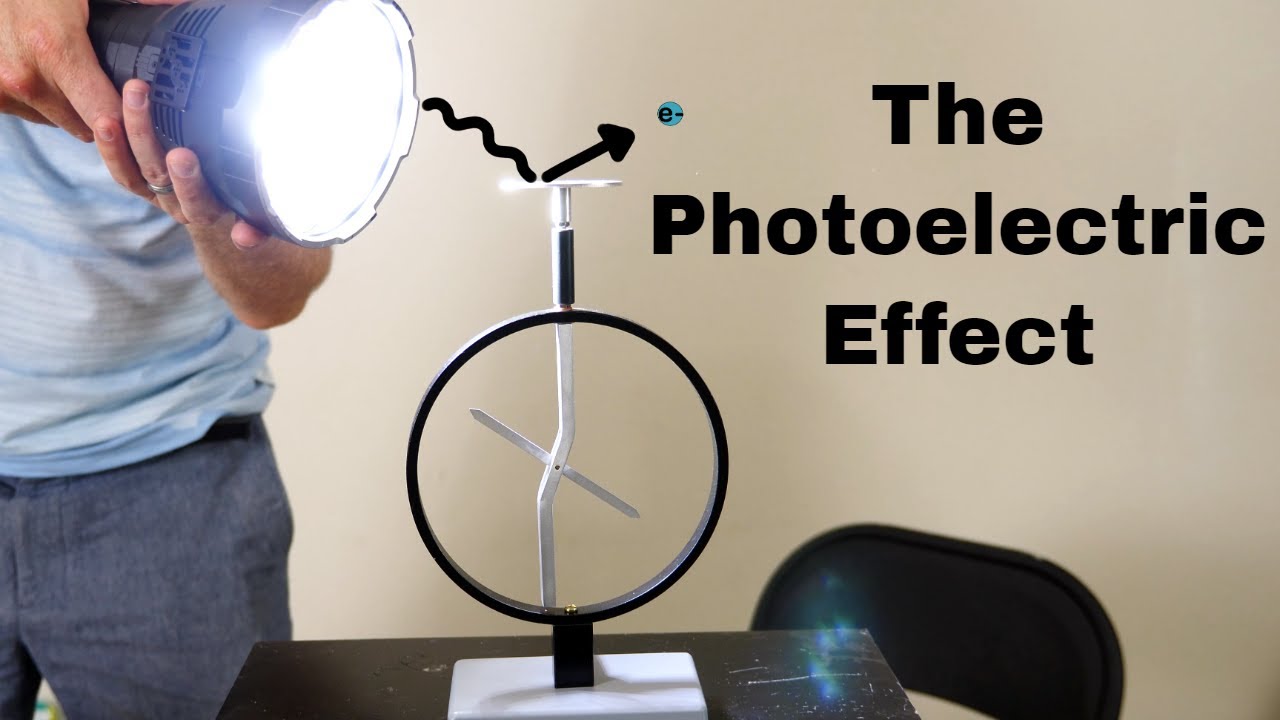
- 😀 The wave theory of light could not explain the photoelectric effect, where light ejects electrons from a metal surface.
- 😀 In the photoelectric effect, light’s ability to eject electrons depends on its frequency, not its intensity. This was a puzzle for scientists.
- 😀 Albert Einstein solved the mystery by applying Max Planck’s concept that energy is quantized (divided into discrete units).
- 😀 Einstein proposed that light is made of particles called photons, which explains the photoelectric effect—one photon with enough energy can eject an electron.
- 😀 The energy of a photon is related to its frequency and Planck’s constant, described by a key equation.
- 😀 Light exhibits wave-particle duality, meaning it behaves both as a wave and as a particle at the same time, marking a major shift in physics and leading to the quantum revolution.
Q & A
What is wave-particle duality?
-Wave-particle duality is the concept that light exhibits both wave-like and particle-like properties. This means that light can behave as a continuous electromagnetic wave in some situations and as a discrete particle, called a photon, in others.
What is the electromagnetic spectrum?
-The electromagnetic spectrum is the range of all types of electromagnetic radiation, from gamma rays to radio waves. Visible light is a small part of this spectrum that can be detected by the human eye.
What is the relationship between wavelength and frequency in light?
-Wavelength and frequency are inversely proportional. The shorter the wavelength, the higher the frequency of the light, and the longer the wavelength, the lower the frequency. This means that as the wavelength decreases, more waves can pass by a point in a given amount of time.
Why could the wave theory of light not explain the photoelectric effect?
-The wave theory of light could not explain the photoelectric effect because it suggested that the energy of light was continuous. However, the photoelectric effect showed that the energy required to eject electrons depended on the frequency of light, not its intensity.
What is the photoelectric effect?
-The photoelectric effect is the phenomenon where light shining on a metal plate ejects electrons from the metal. The ability of light to cause this ejection depends on its frequency, not its intensity, which posed a challenge to classical wave theory.
How did Einstein explain the photoelectric effect?
-Einstein explained the photoelectric effect by suggesting that light is made of particles called photons. Each photon carries a discrete amount of energy, and only photons with enough energy (depending on their frequency) can eject an electron from the metal.
What is a photon?
-A photon is a particle of light, which carries energy quantized in discrete units. The energy of a photon is related to its frequency, and it can interact with matter like other particles.
What did Max Planck contribute to quantum theory?
-Max Planck proposed that energy is quantized, meaning it exists in discrete units rather than continuously. This idea laid the groundwork for quantum theory and helped explain phenomena like the photoelectric effect.
How does quantum theory change our understanding of the universe?
-Quantum theory revolutionized our understanding by showing that many physical quantities, such as energy, space, and time, are quantized. This means that these quantities can’t be divided infinitely and must exist in discrete units, changing how we perceive the microscopic world.
What is the significance of Einstein’s extension of Planck’s ideas?
-Einstein extended Planck's concept of quantized energy to light, proposing that light consists of photons. This explanation helped resolve the issues with the photoelectric effect and marked the beginning of the quantum revolution in physics.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)