Zat dan Perubahannya | IPAS
Summary
TLDRThis educational video explains the physical and chemical properties of matter and how they change. It explores the three states of matter—solid, liquid, and gas—and demonstrates how substances can change states, such as freezing or boiling. The script also covers key physical properties like color, solubility, and conductivity, as well as chemical properties like combustibility, rusting, and toxicity. Through simple experiments and real-life examples, it helps viewers understand how matter behaves, reacts, and transforms in various situations, emphasizing the importance of knowing these properties for everyday life.
Takeaways
- 😀 Different states of matter include solid, liquid, and gas, each with distinct characteristics.
- 😀 Water can change states from liquid to solid (freezing) and liquid to gas (evaporation) depending on temperature.
- 😀 Physical properties of matter include state, color, solubility, electrical conductivity, and magnetism.
- 😀 Chemical properties of matter include flammability, rusting, rotting, explosiveness, and toxicity.
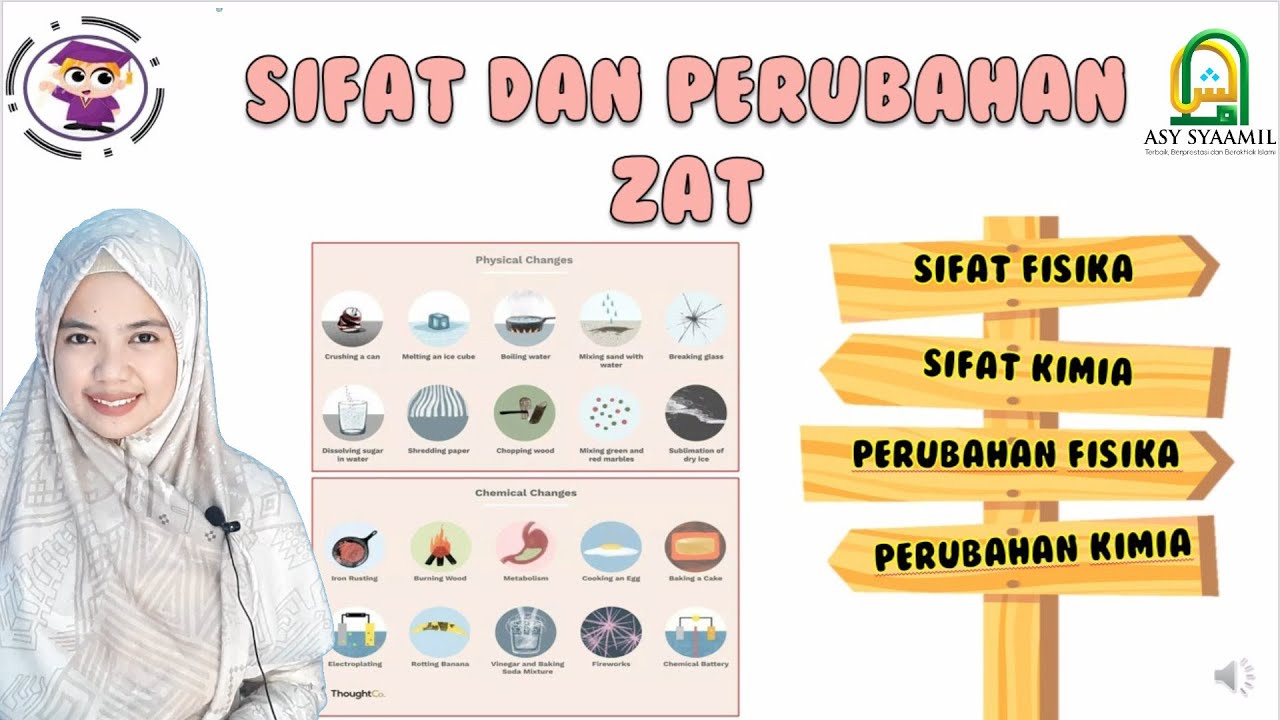
- 😀 Physical changes, like freezing or melting, do not create new substances but only alter the appearance or state.
- 😀 Chemical changes, such as rusting or food rotting, involve the formation of new substances.
- 😀 Rusting occurs when metals like iron react with oxygen and water, leading to corrosion.
- 😀 Not all substances can dissolve in water; for instance, oil does not mix with water, forming two separate layers.
- 😀 Some materials, like metals, can conduct electricity, while others, like rubber, act as insulators.
- 😀 To prevent substances from spoiling, such as food or drinks, we can use preservation methods like refrigeration.
- 😀 Understanding the properties of matter helps us protect health, maintain objects (e.g., preventing rust), and make informed decisions in daily life.
Q & A
What are the effects of changing weather on health?
-Changing weather can cause symptoms like headaches, joint pain, fatigue, and increased susceptibility to colds. This is due to shifts in temperature and humidity, which can affect the respiratory system.
How does rust form on metal, and what does it do?
-Rust forms on metal when it is exposed to both air and water over time. This causes the metal to change color, typically turning reddish or brown, and its surface becomes rough. Eventually, prolonged rusting can cause the metal to weaken, creating holes or breaking it apart.
What is the significance of the experiment with water in three bottles?
-The experiment demonstrates the three physical states of matter: solid, liquid, and gas. By freezing water in one bottle, heating it in another, and leaving the third untreated, we observe how water changes form depending on temperature.
What are the three states of matter?
-The three states of matter are solid, liquid, and gas. Each state is distinguished by the arrangement and movement of particles within the substance.
What is the difference between physical and chemical properties of substances?
-Physical properties relate to the appearance or physical characteristics of a substance, such as color, shape, or state (solid, liquid, gas). Chemical properties involve how a substance reacts with other substances, leading to changes like burning, rusting, or spoiling.
What happens when water freezes or boils in the experiment?
-When water freezes, it changes from liquid to solid, forming ice. When water boils, it transforms from liquid to gas, turning into steam or water vapor.
What are some examples of physical properties of matter?
-Examples of physical properties include appearance (color, texture), solubility (how well a substance dissolves in a solvent like water), electrical conductivity (how well a material conducts electricity), and magnetism (whether a material is attracted to a magnet).
What is the importance of understanding the solubility of substances?
-Understanding solubility is important because it tells us which substances can dissolve in others. For example, salt dissolves in water, while oil does not, forming two separate layers.
How do metals and non-metals differ in terms of electrical conductivity?
-Metals are good conductors of electricity, meaning they allow electricity to pass through them easily. Non-metals, on the other hand, are poor conductors of electricity and are often used as insulators.
What are the two categories of chemical properties discussed in the script?
-The two categories of chemical properties discussed are reactivity and toxicity. Reactivity refers to how substances interact with other materials, such as burning or rusting, while toxicity involves how substances can be harmful or poisonous, such as carbon monoxide.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

Physical vs Chemical Properties - Explained

MACAM ZAT DAN PERUBAHANNYA: IPA KELAS 7 SMP
SIFAT DAN PERUBAHAN ZAT | SIFAT FISIKA DAN KIMIA | PERUBAHAN FISIKA DAN KIMIA

01 02PP01 1StudyofMatter

General Chemistry 1 - Matter and Its Properties

Perubahan Materi / KIMIA kelas 10 kurikulum merdeka , perubahan fisika dan perubahan kimia
5.0 / 5 (0 votes)
