Perubahan Materi / KIMIA kelas 10 kurikulum merdeka , perubahan fisika dan perubahan kimia
Summary
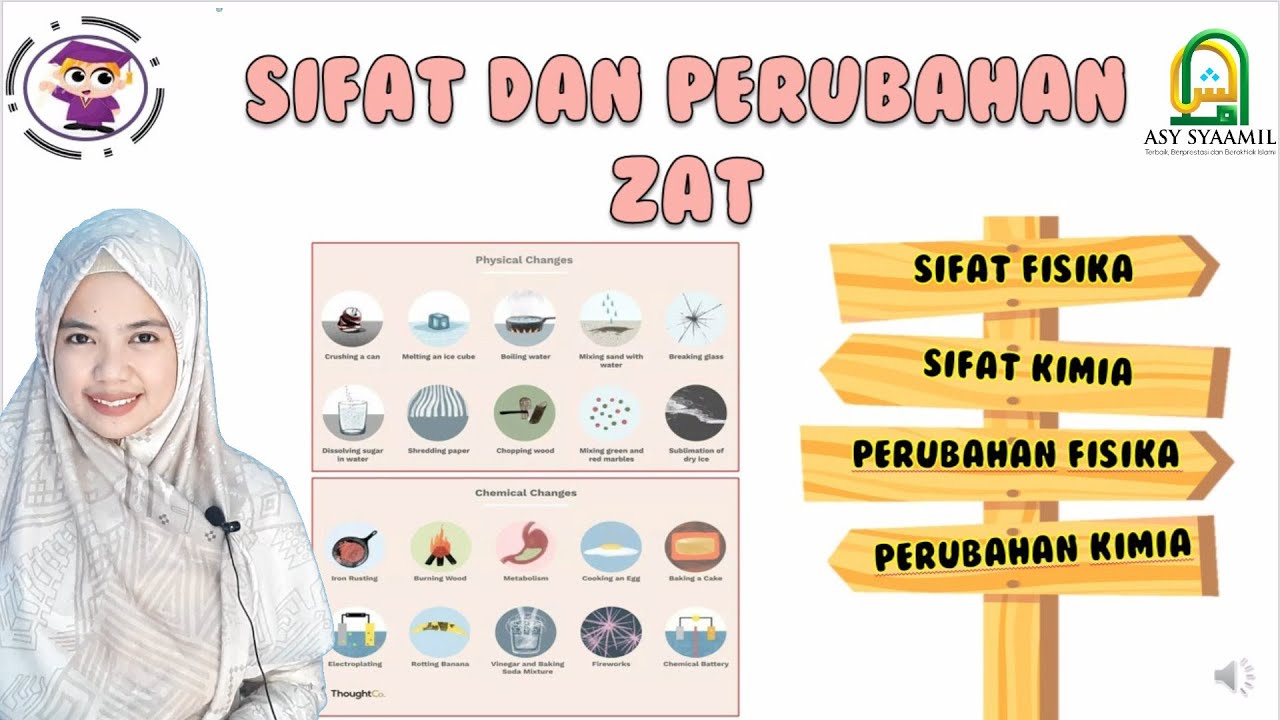
TLDRIn this educational video, viewers learn about the two types of matter changes: physical and chemical. Physical changes, like melting ice or sublimating camphor, do not produce new substances, while chemical changes, such as rusting iron or photosynthesis, result in new substances. The video explains key properties like physical (color, boiling point) and chemical (reactivity, rusting) traits of substances. Through various examples, the video highlights the differences between these types of changes and how to recognize them in everyday life.
Takeaways
- 😀 Physical changes involve changes in the physical state of matter, but the chemical composition remains the same.
- 😀 Examples of physical changes include ice melting, water freezing, and camphor sublimating.
- 😀 Chemical changes result in the formation of new substances with different chemical properties.
- 😀 Photosynthesis is a chemical change, where carbon dioxide and water react to form glucose and oxygen.
- 😀 Rusting of iron is a chemical change, as it produces iron oxide (rust), a new substance.
- 😀 Combustion (burning) is a chemical change, as it generates carbon dioxide and water from materials like paper.
- 😀 Physical changes do not create new substances, they only alter the state or form of a material.
- 😀 Examples of chemical changes include fermentation and decay, where new substances are produced.
- 😀 The key difference between physical and chemical changes is whether a new substance is formed.
- 😀 A quiz in the video asks viewers to identify whether examples are physical or chemical changes, reinforcing the concepts learned.
Q & A
What are the two main types of matter changes discussed in the video?
-The two main types of matter changes discussed are physical changes and chemical changes.
What is the key characteristic of physical properties of matter?
-Physical properties of matter relate to the physical state of a substance and do not involve the formation of a new substance. Examples include color, odor, boiling point, and melting point.
How are physical changes different from chemical changes?
-Physical changes involve changes in the state or appearance of matter without forming a new substance, while chemical changes result in the formation of a new substance.
Can you give an example of a physical change involving water?
-An example of a physical change is ice melting into water. The substance remains H2O, and only the state changes from solid to liquid.
What are some examples of physical changes other than water melting?
-Other examples of physical changes include the sublimation of camphor (solid turning directly into gas) and the freezing of water into ice.
What is a chemical property, and how is it different from a physical property?
-A chemical property relates to the ability of a substance to undergo a chemical reaction and form a new substance. For example, the rusting of iron is a chemical property because it involves the formation of a new substance, rust.
How does rusting illustrate a chemical change?
-Rusting is a chemical change because iron reacts with oxygen and water to form a new substance, iron oxide (rust), which has different properties from the original iron.
What is an example of a chemical reaction that produces a new substance?
-An example is photosynthesis, where carbon dioxide (CO2) and water (H2O) react to form glucose (C6H12O6) and oxygen (O2), creating new substances.
What role do temperature changes play in distinguishing physical and chemical changes?
-Temperature changes can indicate both physical and chemical changes. For instance, a chemical change like combustion often involves a significant temperature rise, while a physical change, such as melting, may involve a temperature change without forming a new substance.
Which of the following is a sign of a chemical change: change in color, change in shape, or change in volume?
-A change in color is often a sign of a chemical change, as it may indicate the formation of a new substance, such as the rusting of iron or the browning of food during cooking.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Physical and Chemical Changes: Chemistry for Kids - FreeSchool

IPA SMA Kelas 10 - Sifat dan Perubahan Materi (Fisika dan Kimia) | GIA Academy

Chemical and Physical Changes – Quiz Edition
SIFAT DAN PERUBAHAN ZAT | SIFAT FISIKA DAN KIMIA | PERUBAHAN FISIKA DAN KIMIA

ZAT DAN PERUBAHANNYA. PROYEK IPAS KELAS X SMK

What Are Physical Properties and Phase Change? | Chemistry Matters
5.0 / 5 (0 votes)