GCSE Chemistry - Percentage Yield #33
Summary
TLDRThis video explains the concept of yield in chemistry, distinguishing between theoretical and actual yield. It covers the common reasons for discrepancies between the expected and actual amount of product, including incomplete reactions, side reactions, and product loss during processing. The video also demonstrates how to calculate percentage yield, a key measure of reaction efficiency, by comparing actual yield with theoretical yield. This overview provides essential insights into understanding chemical reactions and their practical outcomes.
Takeaways
- 😀 The yield in chemistry refers to the amount of product produced in a chemical reaction, and it can be measured in grams or moles.
- 😀 Theoretical yield is the predicted amount of product based on calculations, while actual yield is the real amount obtained after conducting the reaction.
- 😀 Theoretical yield can be determined by adding the weights of reactants. For example, reacting 2 grams of hydrogen with 16 grams of oxygen should give 18 grams of water.
- 😀 Actual yield is often less than the theoretical yield due to factors like incomplete reactions, side reactions, and product loss.
- 😀 Incomplete reactions can happen because the reaction is slow, reversible, or has reached equilibrium, like the ammonia synthesis process.
- 😀 Side reactions occur when reactants form unintended products, such as nitrogen reacting with oxygen to form nitrogen dioxide instead of ammonia.
- 😀 Product loss happens during processes like filtration or if gaseous products escape into the air, reducing the actual amount of product collected.
- 😀 In filtration, some of the liquid or solid may be left behind in the beaker, on the filter paper, or in the residue, leading to a lower yield.
- 😀 The percentage yield is calculated by dividing the actual yield by the theoretical yield and multiplying the result by 100 to express it as a percentage.
- 😀 The percentage yield can range from 0% (no product) to 100% (perfect yield), and it shows the efficiency of the reaction process.
- 😀 For example, if the actual yield is 15 grams and the theoretical yield is 18 grams, the percentage yield would be 83.3%, indicating less than expected was obtained.
Q & A
What is the difference between actual yield and theoretical yield?
-The actual yield is the amount of product that is actually obtained from a chemical reaction, while the theoretical yield is the amount of product that is expected based on calculations, assuming perfect conditions.
Why might the actual yield be less than the theoretical yield in a reaction?
-There are three common reasons: incomplete reactions (where not all reactants are used up), side reactions (where reactants form unintended products), and product loss during the process (such as gas escaping or material left behind during filtration).
Can you explain the concept of reversible reactions in relation to yield?
-In reversible reactions, the reaction doesn't go to completion, and the products can break down back into reactants. This means the reaction reaches an equilibrium state, which results in less product being formed than expected.
What are side reactions, and how do they affect the yield?
-Side reactions occur when reactants form products other than the expected ones. These unintended products reduce the amount of the desired product, resulting in a lower actual yield.
How can the loss of product during a reaction process affect the yield?
-Product loss can happen in several ways, such as gases escaping from the reaction or material being lost during filtration. This reduces the total amount of product recovered, which lowers the actual yield.
What is the purpose of calculating the percentage yield?
-The percentage yield compares the actual yield to the theoretical yield, showing the efficiency of the reaction. It helps to understand how close the reaction comes to producing the expected amount of product.
How do you calculate the percentage yield of a reaction?
-To calculate the percentage yield, divide the actual yield by the theoretical yield and multiply the result by 100. This gives the yield as a percentage of the expected amount.
If a reaction produces 15 grams of product, and the theoretical yield is 18 grams, what is the percentage yield?
-The percentage yield is calculated as (15 ÷ 18) × 100 = 83.3%. This means that 83.3% of the expected product was produced.
What might happen if you don't account for product loss in an experiment?
-If product loss isn't considered, the actual yield will be lower than the theoretical yield, leading to an inaccurate or misleading result when calculating the percentage yield.
What is an example of a situation where you would have incomplete reactions affecting yield?
-An example is the Haber process for ammonia synthesis, where the reaction between nitrogen and hydrogen is reversible. It doesn’t go to completion, so some nitrogen and hydrogen remain unreacted, leading to a lower yield of ammonia.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

How to Calculate Percent Yield
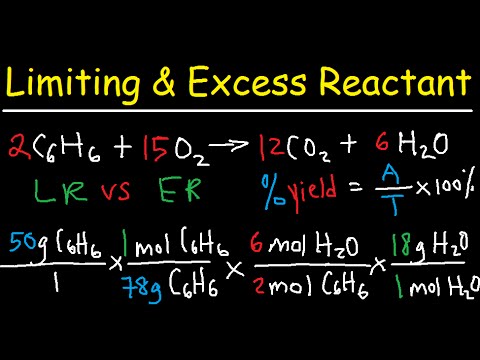
Stoichiometry - Limiting & Excess Reactant, Theoretical & Percent Yield - Chemistry
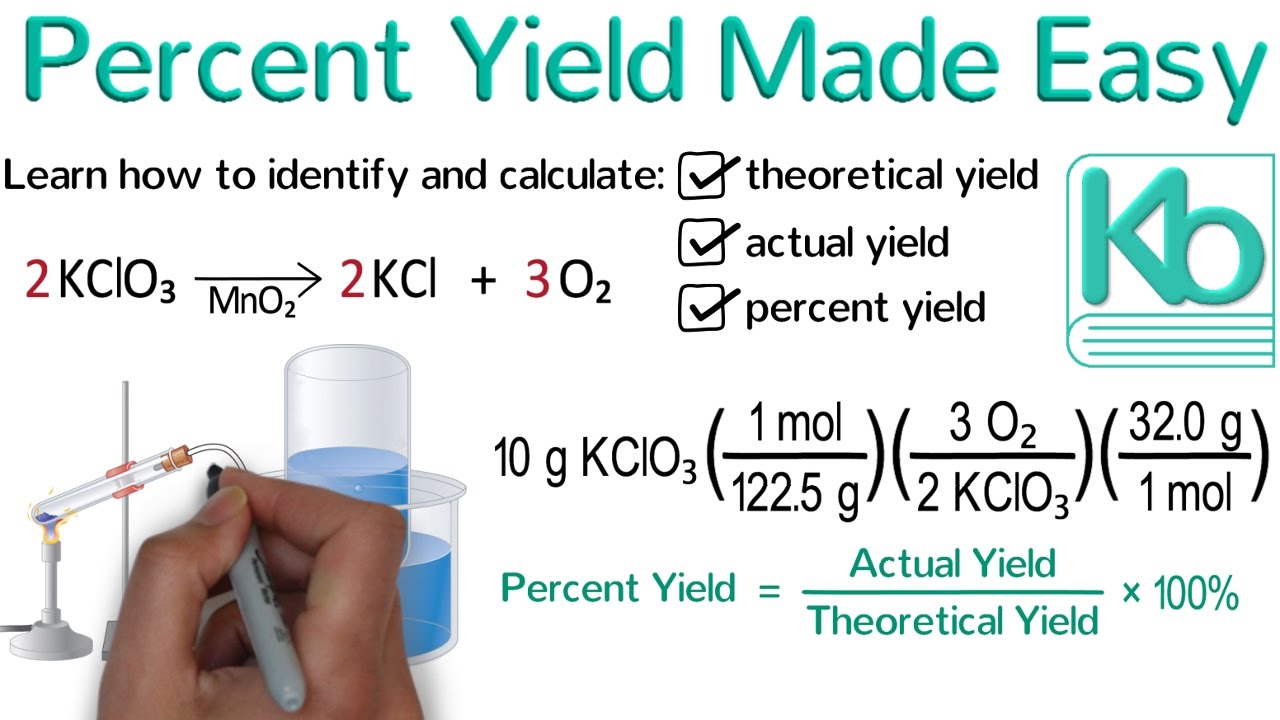
Percent Yield Made Easy: Stoichiometry Tutorial Part 4

9.3 Limiting Reactants and Percentage Yield
03 08PP03 4StoichiometryIntro

Percent Yield for Synthesis of NaCl from Reaction of Sodium Bicarbonate and Hydrochloric Acid
5.0 / 5 (0 votes)
