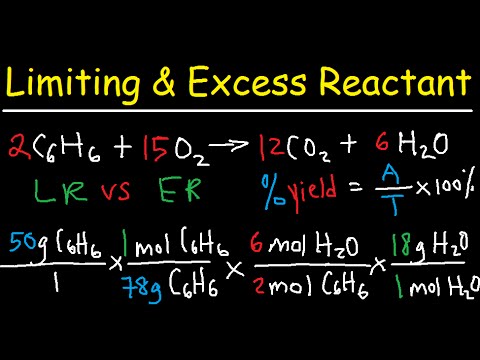Percent Yield Made Easy: Stoichiometry Tutorial Part 4
Summary
TLDRIn this tutorial, we learn how to calculate and distinguish between theoretical yield, actual yield, and percent yield in chemical reactions. Using an analogy of making peanut butter and jelly sandwiches, the video explains the maximum product you can make (theoretical yield), the amount actually made (actual yield), and how to calculate reaction efficiency (percent yield). Through the decomposition of potassium chlorate, the video demonstrates step-by-step how to calculate these yields, while explaining factors that can lead to discrepancies and tips on handling impure products. The tutorial also emphasizes the importance of understanding these concepts in real-life chemical reactions.
Takeaways
- 😀 Theoretical yield is the maximum amount of product that can be made from the available reactants, based on stoichiometry.
- 😀 Actual yield refers to the amount of product actually collected from a reaction.
- 😀 Percent yield is a measure of reaction efficiency and is calculated by dividing the actual yield by the theoretical yield, then multiplying by 100.
- 😀 In the peanut butter and jelly sandwich analogy, the theoretical yield is the maximum sandwiches that could be made, and the actual yield is how many were actually made.
- 😀 To calculate theoretical yield in chemistry, you use stoichiometric conversions, starting with the amount of reactant (not product).
- 😀 Molar mass of reactants and products is essential for stoichiometry calculations.
- 😀 In the potassium chlorate decomposition example, the reaction produces oxygen gas, and the amount of oxygen collected (3.41 g) is the actual yield.
- 😀 Theoretical yield can be calculated by converting grams of reactant to moles, then using the stoichiometric coefficients and molar masses to determine the mass of the product.
- 😀 The percent yield can be calculated by dividing the actual yield by the theoretical yield and multiplying by 100. In the example, the percent yield of oxygen gas was 87%.
- 😀 If the actual yield is less than the theoretical yield, it is normal and can be attributed to incomplete reactions, side reactions, or product loss during collection or purification.
- 😀 If the actual yield exceeds the theoretical yield, it is a sign of an error, such as contamination or incorrect measurements.
Q & A
What is theoretical yield in chemistry?
-Theoretical yield is the maximum amount of product that can be made from the given amount of reactant, assuming everything goes perfectly in the reaction.
What is actual yield in a chemical reaction?
-Actual yield is the amount of product that is actually made and collected in an experiment or reaction. It is typically less than the theoretical yield due to various practical factors.
How do you calculate percent yield?
-Percent yield is calculated by dividing the actual yield by the theoretical yield, then multiplying by 100 to convert it to a percentage.
What is the theoretical yield of oxygen gas in the decomposition of potassium chlorate?
-The theoretical yield of oxygen gas in the decomposition of 10.0 g of potassium chlorate is 3.92 grams.
What factors can cause the actual yield to be less than the theoretical yield?
-Factors like incomplete reactions, side reactions, loss of product due to spillage, sticking to containers, or purification processes can cause the actual yield to be less than the theoretical yield.
What should you do if the actual yield exceeds the theoretical yield?
-If the actual yield exceeds the theoretical yield, it indicates an error in the experiment, such as impurities in the product, measurement mistakes, or calculation errors.
Why is manganese(IV) oxide added to the potassium chlorate decomposition reaction?
-Manganese(IV) oxide is added as a catalyst to speed up the decomposition of potassium chlorate without being consumed in the reaction.
What are the products of the decomposition of potassium chlorate?
-The products of the decomposition of potassium chlorate are potassium chloride (KCl) and oxygen gas (O2).
Why is it important to balance a chemical reaction before performing stoichiometry calculations?
-Balancing the chemical reaction ensures that the law of conservation of mass is followed and allows for accurate stoichiometry calculations, as the number of atoms of each element must be the same on both sides of the reaction.
What could happen if potassium chlorate is exposed to impurities like plastic or rubber?
-Exposing potassium chlorate to impurities like plastic, grease, or rubber can result in a dangerous explosion due to the highly reactive nature of potassium chlorate under certain conditions.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)