9.3 Limiting Reactants and Percentage Yield
Summary
TLDRThis video covers key concepts in chemical reactions, including limiting reactants, excess reactants, theoretical yield, and percentage yield. It explains how to identify the limiting reactant, which dictates the amount of product formed, and demonstrates how to calculate the theoretical yield using stoichiometry. The video also walks through an example problem to calculate percentage yield, comparing actual results with theoretical predictions to assess reaction efficiency. This tutorial is essential for understanding how reactants and yields influence chemical reactions in laboratory settings.
Takeaways
- 😀 Limiting reactants are the substances that are completely consumed in a chemical reaction, dictating how much product can be formed.
- 😀 Excess reactants are the substances that remain after the reaction is complete, in greater quantity than needed for the reaction.
- 😀 Theoretical yield is the maximum amount of product that can be obtained from the reactants, based on stoichiometric calculations.
- 😀 Actual yield refers to the amount of product actually obtained from a reaction in practice, typically measured in the lab.
- 😀 The percentage yield is the ratio of actual yield to theoretical yield, expressed as a percentage, showing the efficiency of a reaction.
- 😀 To determine the limiting reactant, use mole ratios to compare how much product each reactant can produce. The one that produces less is the limiting reactant.
- 😀 In a reaction involving **carbon** and **oxygen**, if you start with 5 moles of carbon and 10 moles of oxygen, carbon will be the limiting reactant.
- 😀 In the example of the reaction between **silicon dioxide** (SiO2) and **hydrofluoric acid** (HF), **hydrofluoric acid** is the limiting reactant when given 6 moles of HF and 4.5 moles of SiO2.
- 😀 The theoretical yield calculation involves using the molar mass of reactants and products and applying the correct mole ratios to predict the maximum product.
- 😀 A common approach to calculating percentage yield involves dividing the actual yield by the theoretical yield, then multiplying by 100 to get a percentage.
Q & A
What is the concept of a limiting reactant in a chemical reaction?
-The limiting reactant is the substance that is entirely consumed in the reaction, limiting the amount of product that can be formed. It is the reactant that runs out first, causing the reaction to stop.
What happens to the excess reactant during a chemical reaction?
-The excess reactant remains after the reaction has completed because there is more of it than is needed to react with the limiting reactant. It does not fully participate in the reaction.
Can you give an example of a limiting reactant from the transcript?
-In the example provided, carbon (C) is the limiting reactant when burned in the presence of oxygen (O₂). Although there are 10 moles of oxygen, only 5 moles of carbon will react, leaving oxygen as the excess reactant.
How is the limiting reactant determined in a reaction?
-The limiting reactant is determined by calculating which reactant produces the least amount of product using stoichiometry and mole ratios. The reactant that produces the least product is the limiting reactant.
What is the theoretical yield of a reaction?
-The theoretical yield is the maximum amount of product that can be produced in a reaction based on the amounts of reactants and the stoichiometry of the reaction.
What is the actual yield of a chemical reaction?
-The actual yield is the amount of product actually obtained from a reaction after it is completed, measured experimentally.
Why do actual yields often differ from theoretical yields?
-Actual yields often differ from theoretical yields due to factors such as incomplete reactions, side reactions, impurities, or losses during product isolation and handling.
What is percentage yield and how is it calculated?
-Percentage yield is a measure of the efficiency of a reaction, calculated as the ratio of the actual yield to the theoretical yield, multiplied by 100 to express it as a percentage.
How is stoichiometry used to determine the limiting reactant?
-Stoichiometry uses mole ratios derived from the balanced chemical equation to calculate how much product can be formed from each reactant. The reactant that produces the least product is the limiting reactant.
In the example with benzene and chlorobenzene, what is the theoretical yield and percentage yield?
-In the example, the theoretical yield of chlorobenzene is calculated as 53.0 grams. The percentage yield is calculated by dividing the actual yield (38.8 grams) by the theoretical yield (53.0 grams) and multiplying by 100, giving a percentage yield of 73.2%.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
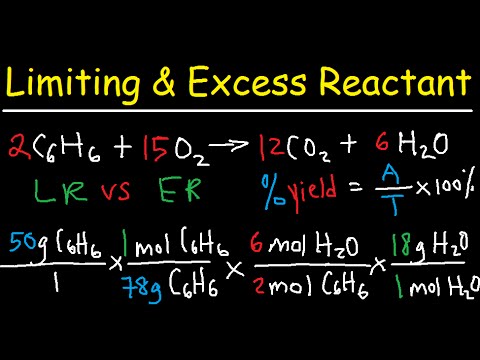
Stoichiometry - Limiting & Excess Reactant, Theoretical & Percent Yield - Chemistry

QUANTITATIVE CHEMISTRY - GCSE Chemistry (AQA Topic C3)

Introduction to Limiting Reactant and Excess Reactant

GCSE Chemistry - Percentage Yield #33

Stoichiometry

IB Chemistry Topic 1 Stoichiometric relationships Topic 1.3 Reacting masses and volumes SL
5.0 / 5 (0 votes)