Introduction to Limiting Reactant and Excess Reactant
Summary
TLDRThis video explains the concepts of limiting reactants and excess reactants using relatable cooking analogies, such as making cheeseburgers and bread rolls. It highlights how the limiting reactant is the ingredient that runs out first, dictating the maximum product yield. By analyzing a chemical equation involving nitrogen and hydrogen, the video further demonstrates how to identify the limiting reactant through stoichiometric calculations. Viewers will gain a clear understanding of these key chemistry concepts and how to apply them in problem-solving.
Takeaways
- 😀 Limiting reactants are the first ingredients used up in a chemical reaction, determining the maximum product yield.
- 🍔 Cooking analogies help illustrate chemical concepts, like comparing reactants to ingredients in a recipe.
- 🥩 In the cheeseburger example, meat is the limiting reactant; even with extra buns and cheese, no more burgers can be made without meat.
- 🍞 Excess reactants are the ingredients left over after the reaction has stopped because the limiting reactant is depleted.
- 🔍 Identifying the limiting reactant involves calculating the amount of product that can be formed based on available ingredients.
- 📊 For bread rolls, starting with different reactants helps determine which is limiting by checking how much of the other is needed.
- ⚗️ In chemical equations, coefficients indicate the ratios of reactants and products, crucial for stoichiometric calculations.
- 🧮 Using conversion factors allows for systematic determination of required amounts of reactants in reactions.
- 🚫 A common misconception is that the limiting reactant is the one present in the smallest quantity; it’s actually the one that runs out first.
- 📚 Practice with various examples is essential to mastering the concept of limiting and excess reactants in chemistry.
Q & A
What is a limiting reactant?
-A limiting reactant is the reactant that is used up first in a chemical reaction, determining the maximum amount of product that can be formed.
How does the analogy of cooking help explain limiting and excess reactants?
-The cooking analogy illustrates how different ingredients (reactants) can limit the number of final products (like cheeseburgers) based on which ingredient is used up first.
In the cheeseburger example, which ingredient was the limiting reactant?
-In the cheeseburger example, the piece of meat was the limiting reactant since it was used up first, preventing more cheeseburgers from being made.
What is an excess reactant?
-An excess reactant is any reactant that remains after the limiting reactant has been completely consumed in a reaction.
Why is it a misconception that the limiting reactant is always the one present in the least amount?
-This misconception arises because the limiting reactant is defined by its ability to be used up first in the reaction, not simply by its initial quantity.
How do you determine which reactant is limiting when given amounts of two reactants?
-To find the limiting reactant, calculate how much of the second reactant is needed for each reactant when using them to their maximum capacity, then compare available amounts.
What method was used to solve the bread rolls example?
-In the bread rolls example, the approach involved checking how much of each ingredient (flour and water) was needed when maximizing the use of one ingredient at a time.
What was the limiting reactant in the bread rolls example?
-In the bread rolls example, flour was the limiting reactant because it was used up before the water, limiting the number of bread rolls that could be made.
How do you calculate the amount of product that can be made from the limiting reactant?
-To calculate the amount of product, multiply the moles of the limiting reactant by the appropriate stoichiometric coefficient from the balanced chemical equation.
What is the significance of understanding limiting and excess reactants in chemistry?
-Understanding limiting and excess reactants is crucial for predicting the amounts of products formed in chemical reactions, optimizing reactant usage, and minimizing waste.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

9.3 Limiting Reactants and Percentage Yield

R2.1.2 Molar ratio
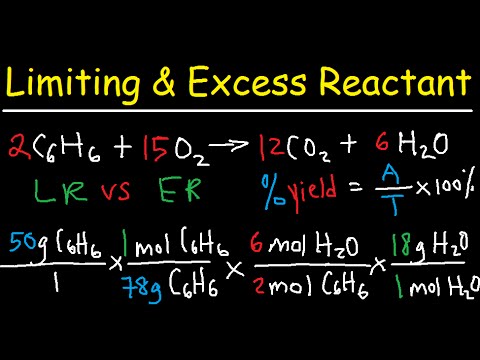
Stoichiometry - Limiting & Excess Reactant, Theoretical & Percent Yield - Chemistry

GCSE Chemistry - What is a Limiting Reactant? Limiting/Excess Reactants Explained #27

IB Chemistry Topic 1 Stoichiometric relationships Topic 1.3 Reacting masses and volumes SL

Stoikiometri (6) | Pereaksi Pembatas | Kimia Kelas 10
5.0 / 5 (0 votes)
