ATOMIC MODELS: Democritus, Dalton, Thomson, Rutherford, Bohr, Sommerfeld, Quantum
Summary
TLDRThe history of atomic models spans from ancient Greek philosophical ideas to modern scientific discoveries. Early thinkers like Democritus imagined the atom as indivisible, but later scientists, including John Dalton, Joseph Thomson, and Ernest Rutherford, introduced more refined models based on experimental findings. Niels Bohr further developed the model with electron orbits, while Arnold Sommerfeld expanded it using relativity. Finally, the quantum mechanical model, developed by Heisenberg, Schrödinger, and de Broglie, replaced the concept of orbits with electron clouds and introduced the probabilistic nature of electron positions, cementing our modern understanding of atomic structure.
Takeaways
- 😀 Atomic models are graphical representations that explain the structure and function of atoms, which have evolved over time as scientific knowledge advanced.
- 😀 The concept of atoms dates back to ancient Greece, where it was initially a philosophical idea before becoming scientifically understood.
- 😀 Atoms are the fundamental building blocks of matter, making up everything from our bodies to large structures like skyscrapers and even celestial bodies like the Sun and Moon.
- 😀 Despite being extremely small, atoms are not the smallest units in nature, as they are made up of protons, neutrons, and electrons.
- 😀 Democritus, an ancient Greek philosopher, first proposed that matter is made up of indivisible particles called 'atoms,' which are eternal, indestructible, and compact.
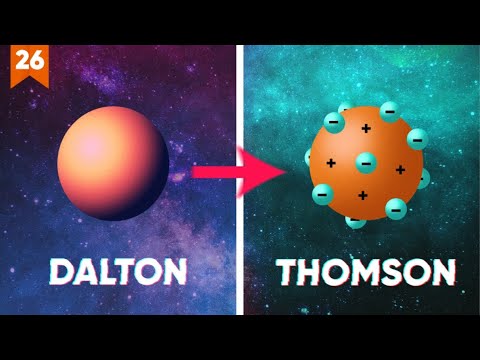
- 😀 John Dalton's model (1803) depicted atoms as indivisible and solid spheres, marking the first modern atomic theory, prior to the discovery of subatomic particles.
- 😀 Joseph John Thomson's model (1904), known as the 'raisin pudding' model, placed electrons within a positively charged 'pudding,' addressing the discovery of the electron.
- 😀 Ernest Rutherford's model (1911) proposed a tiny, dense nucleus with electrons orbiting around it, likened to a miniature solar system, based on his gold foil experiment.
- 😀 Niels Bohr's atomic model (1913) introduced stable circular orbits for electrons, with energy levels separating these orbits, solving issues in Rutherford's model.
- 😀 Arnold Sommerfeld extended Bohr's model (1916) by including elliptical orbits and energy sublevels, drawing from Einstein's theory of relativity.
- 😀 The Quantum Mechanical Model, developed by Heisenberg, Schrödinger, and de Broglie, replaces orbits with electron clouds, using probability to describe electron positions, and introduces quantum numbers to explain electron behavior.
Q & A
What was the original concept of the atom according to ancient Greek philosophers?
-The original concept of the atom, according to ancient Greek philosophers like Democritus, was that it was an indivisible particle that made up all matter. These early ideas were philosophical speculations rather than scientific theories.
How did the discovery of protons, neutrons, and electrons shape the atomic model?
-The discovery of protons, neutrons, and electrons helped develop more accurate atomic models. These particles revealed that atoms were not indivisible, but made up of smaller components. The electron, discovered by Thomson, led to a rethinking of the atom's structure, where electrons orbit a nucleus.
What was the major contribution of John Dalton to atomic theory?
-John Dalton's major contribution in 1803 was the first modern atomic model. He proposed that atoms were indivisible and solid spheres. Dalton also introduced the idea that atoms of different elements are distinct in size and mass, and that chemical reactions involve the rearrangement of atoms.
Why was Thomson's 'raisin pudding' model significant?
-Thomson's 'raisin pudding' model, proposed in 1904, was significant because it introduced the idea of the electron as a negatively charged particle embedded in a positively charged atom. This model was a key step in understanding atomic structure, even though it was later replaced by Rutherford's and Bohr's models.
How did Rutherford’s experiment change the understanding of the atom?
-Rutherford's gold foil experiment in 1911 showed that the atom has a small, dense, positively charged nucleus, which contradicted Thomson's 'raisin pudding' model. His discovery led to the concept of a nuclear model, where electrons orbit around a central nucleus.
What issue with Rutherford's model did Niels Bohr address?
-Niels Bohr addressed the problem of electrons losing energy and collapsing into the nucleus in Rutherford's model. Bohr proposed that electrons occupy stable, fixed orbits with specific energy levels, preventing them from spiraling into the nucleus.
What was the main modification made by Arnold Sommerfeld to Bohr's model?
-Arnold Sommerfeld extended Bohr's model by introducing elliptical orbits and recognizing sublevels within energy levels. He also incorporated relativistic effects, acknowledging that some electrons move close to the speed of light.
How does the Quantum Mechanical Model differ from earlier atomic models?
-The Quantum Mechanical Model, developed by Heisenberg, Schrödinger, and de Broglie, differs from earlier models by eliminating fixed electron orbits. Instead, electrons exist as probability clouds around the nucleus, and their positions are determined by wave equations, allowing for a more accurate description of atomic behavior.
What role do quantum numbers play in the Quantum Mechanical Model?
-In the Quantum Mechanical Model, quantum numbers define the specific state of each electron in an atom. These include the main quantum number, secondary quantum number, magnetic quantum number, and spin quantum number, which together describe an electron's energy, shape of its orbit, orientation, and spin.
Why did Democritus' atomic theory remain largely forgotten until modern science?
-Democritus' atomic theory was largely forgotten because it was based on philosophical speculation rather than empirical evidence. It was not until the development of modern scientific techniques that the concept of the atom was revived and supported by experimental data.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

Química - Natureza elétrica da matéria e núcleo atômico (prof. Luiz Landim)

Review of: History of an atom & Ions

MODELOS ATÔMICOS: Dalton, Thomson, Rutherford e Rutherford-Bohr

IPA Kelas 9 Semester 2 : Partikel Penyusun Materi (Part 1 : atom dan molekul)
O Modelo Atômico de Dalton x Thomson

All Chemistry Explained in 17 Minutes
5.0 / 5 (0 votes)
