Gay Lussac's Law Practice Problems
Summary
TLDRThis tutorial explains Gay-Lussac's Law, which describes the relationship between pressure and temperature in a rigid container. It demonstrates that as the temperature of a gas increases, so does the pressure, as faster-moving molecules collide more frequently with the container walls. The video covers practical examples, such as heating water in a pot and how pressure builds up, eventually lifting the lid. The law's formula (P1/T1 = P2/T2) is applied to practice problems, including calculating pressure changes with temperature variations and converting units to ensure accurate results.
Takeaways
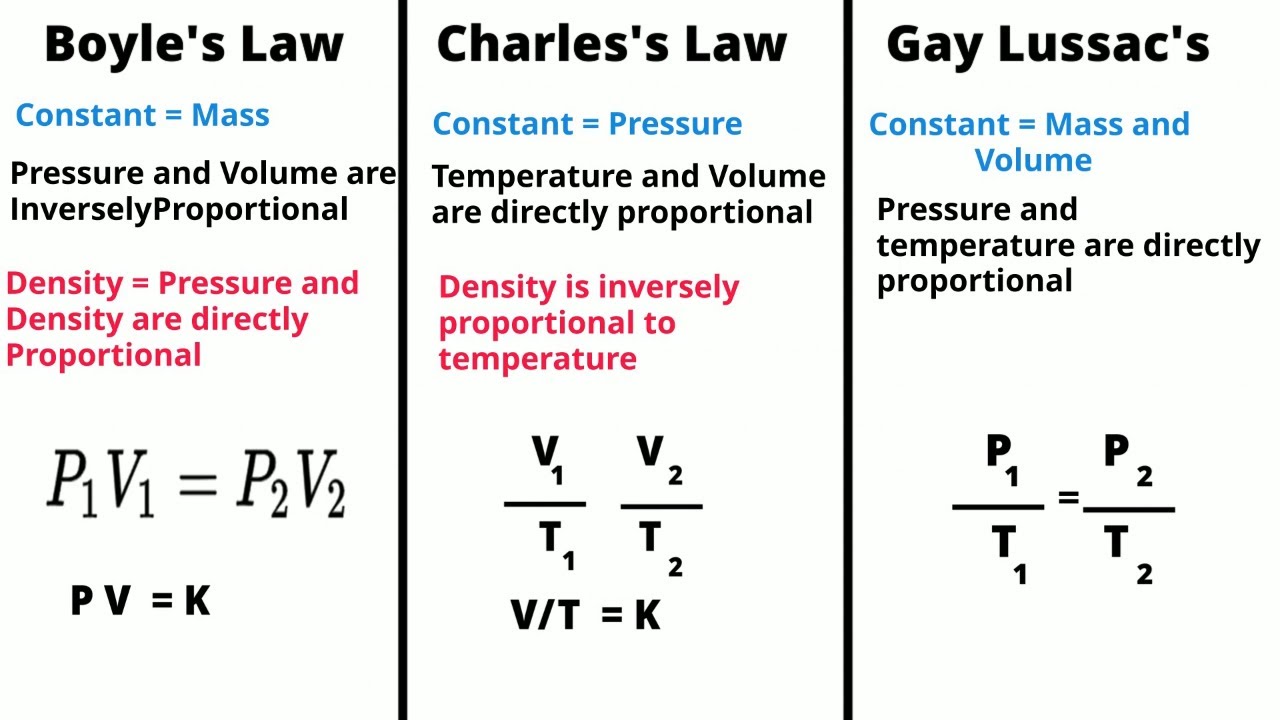
- 😀 Gay Lussac's Law states that the pressure of a gas is directly proportional to its temperature when volume is constant.
- 😀 If the temperature of a gas increases, its molecules move faster, leading to more frequent collisions with the container walls, which increases the pressure.
- 😀 The law applies to rigid containers where the volume does not change. If the volume changes, the relationship between temperature and pressure may differ.
- 😀 The formula for Gay Lussac's Law is P1/T1 = P2/T2, where the temperatures must be in Kelvin, not Celsius.
- 😀 When using Gay Lussac's Law, the pressure should be in consistent units, such as Torr, atm, or kPa, to ensure accurate calculations.
- 😀 An example of Gay Lussac's Law in action is heating water in a pot. As the water vaporizes and the temperature increases, the pressure builds, potentially lifting the pot lid.
- 😀 A real-world application of Gay Lussac's Law is the increase in tire pressure when the tires heat up during driving due to the rise in temperature.
- 😀 In practice, if temperature increases by a factor of three, pressure should also increase by a factor of three, as seen in the calculation example from 125 kPa to 375 kPa.
- 😀 When solving problems, convert Celsius to Kelvin by adding 273, as the formula requires temperatures in Kelvin for consistency.
- 😀 It's important to ensure unit consistency when working with pressure and temperature in Gay Lussac's Law. If pressure is in psi, the result will also be in psi, but temperature must always be in Kelvin.
- 😀 In a problem involving gas pressure, when pressure decreases, the temperature is expected to decrease as well, which is confirmed through calculations that showed a decrease in temperature after a drop in pressure.
Q & A
What is Gay-Lussac's Law?
-Gay-Lussac's Law states that the pressure of a gas is directly proportional to its temperature when the volume is held constant.
How does increasing the temperature of a gas affect its pressure in a rigid container?
-Increasing the temperature of a gas in a rigid container increases the kinetic energy of the gas molecules, causing them to move faster and collide more frequently with the container walls. This leads to an increase in pressure.
What happens if the volume of the container is allowed to expand while heating the gas?
-If the volume is allowed to expand while heating the gas, the pressure could remain constant, as the volume compensates for the temperature increase.
How does the number of molecular collisions relate to the pressure in a container?
-The more frequently gas molecules collide with the walls of the container, the higher the pressure inside. This is because collisions exert force on the walls, increasing the pressure.
What is the equation associated with Gay-Lussac's Law?
-The equation for Gay-Lussac's Law is P1/T1 = P2/T2, where P represents pressure, T represents temperature (in Kelvin), and the subscripts 1 and 2 represent initial and final conditions.
Why must temperature be measured in Kelvin for calculations involving Gay-Lussac's Law?
-Temperature must be in Kelvin because the Kelvin scale starts at absolute zero, which is a theoretical point where molecular motion stops. Using Kelvin ensures the temperature is always a positive value and correctly proportional in calculations.
What happens to the pressure of a gas when its temperature increases from 300 K to 900 K?
-If the temperature increases from 300 K to 900 K, the pressure will also increase proportionally. Specifically, the pressure will triple because the temperature has tripled.
How do you calculate the new pressure when the temperature changes, using Gay-Lussac's Law?
-To calculate the new pressure, use the equation P1/T1 = P2/T2. Rearrange the equation to solve for P2, then cross-multiply and divide to find the new pressure.
In the practice example with the tire pressure, what units were used to calculate the pressure change?
-In the tire pressure example, the pressure was measured in pounds per square inch (psi), and the temperatures were converted to Kelvin to apply Gay-Lussac's Law.
What happens to the pressure in the pot example when heating water in a sealed container?
-As the water is heated, some of the water vaporizes into steam, causing the pressure inside the sealed pot to increase. If the pressure gets too high, it can lift the lid of the pot to relieve the pressure.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
5.0 / 5 (0 votes)