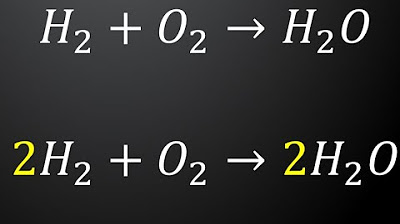PERSAMAAN REAKSI DAN CARA PENYETARAANNYA ( KIMIA SMA KELAS 10 )
Summary
TLDRThis educational script explains the process of writing and balancing chemical equations in chemistry. It covers the key concepts, such as the symbols for different states of matter (gas, liquid, solid, aqueous), the role of coefficients in balancing equations, and how to represent chemical reactions correctly. The script walks through multiple examples, demonstrating how to balance equations and ensure the number of atoms on both sides are equal. The goal is to help students understand the basics of chemical reactions and the importance of precision in writing balanced equations.
Takeaways
- 😀 In chemistry, the primary focus is studying the changes in matter or chemical reactions.
- 😀 Chemical reactions are represented using chemical symbols and formulas in chemical equations.
- 😀 Reactants (substances that undergo the reaction) are written on the left, and products (the outcome of the reaction) are written on the right of the equation.
- 😀 The arrow (→) in the chemical equation indicates the direction of the chemical reaction.
- 😀 There are four states of matter that should be included in the chemical equation: gas (g), liquid (l), solid (s), and aqueous (aq).
- 😀 Coefficients in front of chemical formulas indicate the ratio of particles involved in the reaction.
- 😀 A chemical reaction is considered balanced when the number of atoms on both sides of the equation is equal.
- 😀 To balance a chemical equation, the coefficients of the reactants and products are adjusted until atom counts on both sides match.
- 😀 Example: The reaction of hydrogen and oxygen to form water is balanced with the equation 2 H₂ + O₂ → 2 H₂O.
- 😀 In a reaction like methane combustion, the chemical equation CH₄ + 2 O₂ → CO₂ + 2 H₂O needs to be balanced by adjusting the coefficients of the products.
- 😀 Chemical equations can involve simple reactions (like metal and acid reactions) or complex ones involving gases and solutions (e.g., aluminum and hydrochloric acid producing hydrogen gas).
Q & A
What is the purpose of chemical equations in representing chemical reactions?
-Chemical equations are used to represent chemical reactions by showing the reactants on the left and the products on the right, helping to communicate the reaction clearly and succinctly.
What are the main components of a chemical equation?
-A chemical equation consists of reactants, products, coefficients, and phase indicators (solid, liquid, gas, or aqueous). Reactants are on the left, and products are on the right, connected by an arrow to indicate the direction of the reaction.
What do the coefficients in a chemical equation represent?
-Coefficients in a chemical equation indicate the simplest whole number ratio of particles (atoms or molecules) involved in the reaction. They balance the number of atoms on both sides of the equation.
What does it mean for a chemical equation to be 'balanced'?
-A chemical equation is considered balanced when the number of atoms for each element is the same on both sides of the equation, ensuring mass conservation.
How do you indicate the physical states of the substances in a chemical equation?
-The physical states of substances are indicated by symbols in parentheses: (g) for gas, (l) for liquid, (s) for solid, and (aq) for aqueous solutions (substances dissolved in water).
In the reaction 2 H₂(g) + O₂(g) → 2 H₂O(l), what is the role of the coefficients?
-In this reaction, the coefficients (2 in front of H₂ and H₂O) indicate that two molecules of hydrogen gas react with one molecule of oxygen gas to form two molecules of water.
Why is it unnecessary to write the coefficient '1' in a balanced equation?
-In a balanced chemical equation, a coefficient of '1' is implied and does not need to be written explicitly. For example, 'O₂' implies one molecule of oxygen without needing the '1' in front.
What does the symbol '(aq)' indicate in a chemical equation?
-'(aq)' stands for aqueous, meaning the substance is dissolved in water. It indicates that the substance is in a solution form.
What is the general process for balancing a chemical equation?
-To balance a chemical equation, you adjust the coefficients in front of the reactants and products until the number of atoms for each element is the same on both sides of the equation.
In the example 2 Na(s) + 2 H₂O(l) → 2 NaOH(aq) + H₂(g), what are the reactants and products?
-In this reaction, the reactants are sodium (Na) and water (H₂O), and the products are sodium hydroxide (NaOH) and hydrogen gas (H₂).
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآن5.0 / 5 (0 votes)