Tetapan Kesetimbangan Berdasarkan Tekanan (Kp) | Kimia SMA Kelas 11
Summary
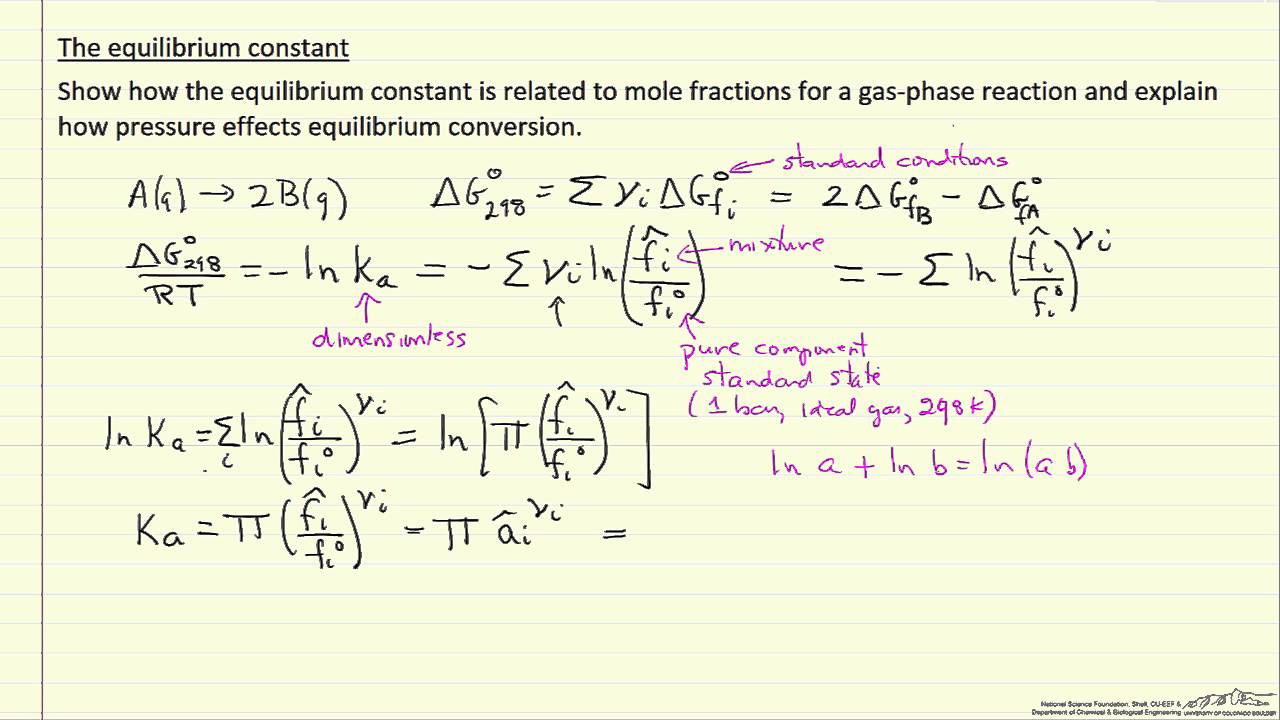
TLDRThis video explains the concept of equilibrium constant (KP) in chemistry, focusing on how it is influenced by pressure and the gas phase. The presenter demonstrates the process of calculating KP with real-life examples, including the decomposition of ammonia and the dissociation of lanthanum oxalate. Key concepts covered include the importance of gas phases in KP calculations and how to convert moles into pressure values to find the equilibrium constant. The video concludes with a second example, reinforcing the steps and offering helpful tips for solving such problems in chemistry.
Takeaways
- 😀 KP equilibrium constant is influenced only by gases, represented as G in chemical reactions.
- 😀 When calculating KP, the phase of the substance is important—solids and liquids do not affect the calculation.
- 😀 In reactions involving gases, the KP formula is the product of partial pressures raised to the power of their respective coefficients.
- 😀 Example: In the reaction 2SO2 + O2 ⇌ 2SO3, the KP is calculated as (Pressure of SO3)^2 / (Pressure of SO2)^2 * (Pressure of O2).
- 😀 When a solid phase is involved in equilibrium, it is excluded from the KP expression, as only gases contribute.
- 😀 For calculating KP, it’s important to convert mole quantities to partial pressures using the formula: P = (moles of gas / total moles of gas) * total pressure.
- 😀 In the given example, the decomposition of NH3 into N2 and H2 is discussed, with a focus on how to calculate KP using pressures and mole ratios.
- 😀 The process of converting moles to pressures is demonstrated using the equilibrium data for NH3, N2, and H2, with specific pressures calculated for each.
- 😀 The second example demonstrates how to handle a more complex equilibrium involving solid and gas phases. Here, only the gases CO and CO2 are considered for KP calculation.
- 😀 The final calculation of KP involves raising the partial pressures of CO and CO2 to the power of their coefficients (both 3), resulting in a KP value of 10^-6.
Q & A
What is the equilibrium constant (KP) and how is it determined?
-The equilibrium constant (KP) is a value that describes the ratio of the partial pressures of products to reactants in a chemical reaction at equilibrium. It is determined by taking the product of the pressures of the gaseous products, each raised to the power of their respective coefficients, and dividing that by the product of the pressures of the gaseous reactants, each raised to the power of their coefficients.
Which phases of substances are considered in the calculation of KP?
-Only substances in the gaseous phase are considered in the calculation of KP. Solids and liquids do not appear in the expression for KP because their concentrations do not change during the reaction.
Why is the phase of the substance important in KP calculations?
-The phase is important because KP only includes the pressures of gases. Solids and liquids are not included since their activities are constant and do not change during the reaction, hence they do not affect the equilibrium constant.
How does the presence of solids affect the calculation of KP in a reaction?
-Solids are not included in the KP expression because their concentration remains constant during the reaction. Only gases are included in the KP calculation because their pressures change during the reaction.
In the first example, how did the molar amounts of NH₃, N₂, and H₂ at equilibrium help calculate KP?
-In the first example, the moles of NH₃, N₂, and H₂ at equilibrium were used to calculate the partial pressures of each gas. These pressures were then used in the KP expression to determine the equilibrium constant.
What steps were involved in determining the partial pressures in the first example?
-First, the moles of each gas at equilibrium were used to calculate their respective partial pressures by using the formula: partial pressure = (moles of gas / total moles of gas) × total pressure. This was done for NH₃, N₂, and H₂.
How do the coefficients of the chemical equation affect the KP calculation?
-The coefficients of the chemical equation determine the exponents to which the partial pressures are raised. For example, if the coefficient of a substance is 2, its partial pressure is raised to the power of 2 in the KP expression.
Why is the total pressure used to calculate partial pressures in the first example?
-The total pressure is used because it represents the combined effect of all the gases in the system. The partial pressure of each gas is calculated based on its mole fraction in relation to the total moles of gas in the system.
In the second example, why are only the pressures of CO and CO₂ considered in the KP expression?
-In the second example, only CO and CO₂ are considered in the KP expression because they are the only gases in the reaction. The solid substances (La₂(C₂O₄)₃ and La₂O₃) are not included as they do not affect the equilibrium constant.
What is the significance of the total pressure in the second example?
-In the second example, the total pressure provides the necessary information to calculate the partial pressures of CO and CO₂. Since the total pressure is given, the mole fractions of CO and CO₂ can be used to calculate their individual partial pressures.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
5.0 / 5 (0 votes)