Kesetimbangan Kimia • Part 1: Konsep, Hukum, Tetapan Kesetimbangan Kc dan Kp
Summary
TLDRThis educational video explains the concept of chemical equilibrium, focusing on reversible and irreversible reactions. It covers key topics such as equilibrium constants (Kc and Kp), dynamic equilibrium, and how external factors can affect equilibrium states. The video provides practical examples like ammonia synthesis and acid-base equilibrium, illustrating how to calculate equilibrium constants using concentration and pressure. It also distinguishes between homogeneous and heterogeneous equilibria, emphasizing the importance of understanding the phases involved in each reaction. Overall, it serves as a comprehensive guide to mastering chemical equilibrium in high school chemistry.
Takeaways
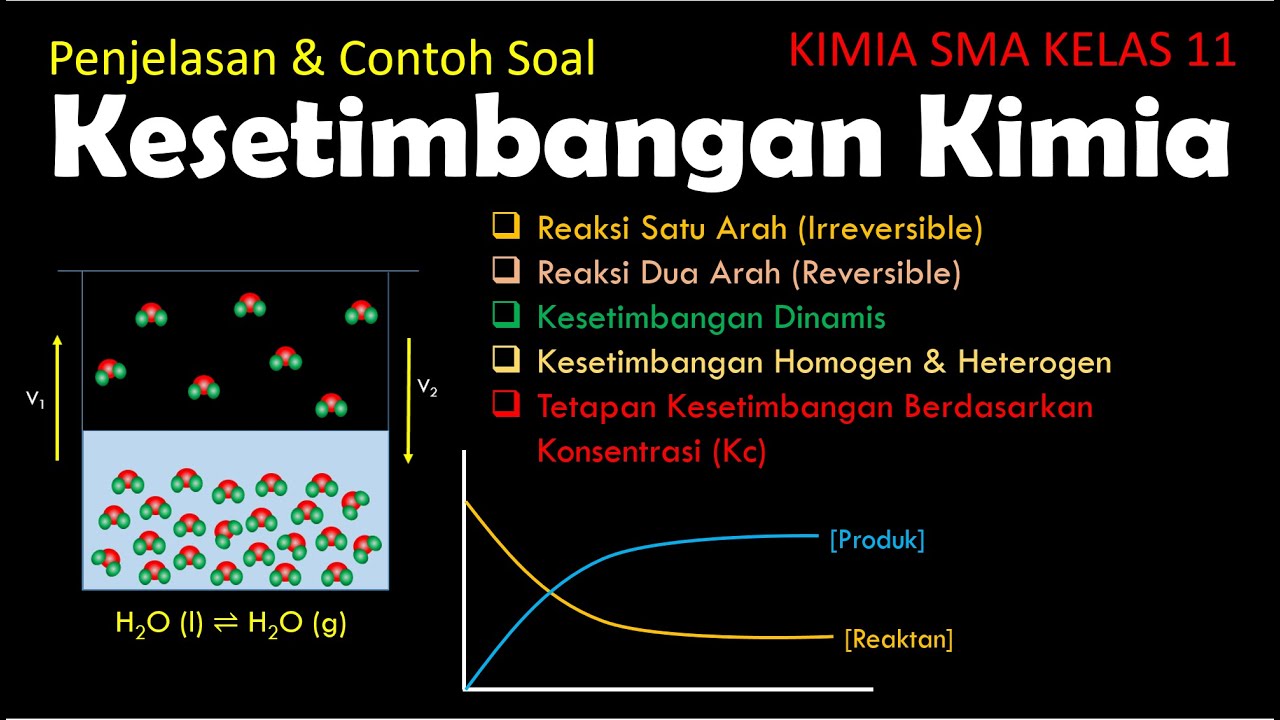
- 😀 Irreversible reactions only proceed in one direction, and products cannot revert back to reactants.
- 😀 Reversible reactions can proceed in both directions, with products potentially reacting back to form reactants, reaching a dynamic equilibrium.
- 😀 Dynamic equilibrium is when the forward and reverse reactions occur at the same rate, maintaining constant concentrations of reactants and products.
- 😀 Reactions can be classified into two types: homogeneous equilibrium (all components in the same phase) and heterogeneous equilibrium (components in different phases).
- 😀 The equilibrium constant (K) represents the ratio of concentrations of products to reactants at equilibrium, raised to their respective coefficients.
- 😀 In equilibrium constant expressions (Kc), solids and liquids are excluded because they do not have a concentration that can change during the reaction.
- 😀 The equilibrium constant for gas-phase reactions can be expressed using partial pressures (Kp), where pressure replaces concentration in the formula.
- 😀 For reversible reactions, the concentration of reactants and products stabilizes, but molecular reactions continue to occur in both directions.
- 😀 The equilibrium constant formula involves products over reactants, with each concentration raised to the power of its coefficient in the balanced equation.
- 😀 When calculating Kc and Kp, gases and aqueous solutions are included, but solids and liquids are excluded, since their concentrations remain constant in equilibrium.
- 😀 The relationship between Kc and Kp is given by the equation: Kp = Kc * (RT)^Δn, where R is the gas constant, T is the temperature in Kelvin, and Δn is the change in the number of moles of gas.
Q & A
What is the difference between irreversible and reversible reactions?
-Irreversible reactions proceed in only one direction, where reactants form products that cannot revert to the original reactants. An example is the combustion of methane. In contrast, reversible reactions occur in both directions, where products can decompose back into reactants, such as the formation of ammonia from nitrogen and hydrogen gases.
Why can't irreversible reactions reach chemical equilibrium?
-Irreversible reactions do not reach equilibrium because once the reactants are converted to products, they cannot reverse back into reactants. This means the reaction eventually completes and stops, with no further changes in the concentrations of the reactants or products.
What is dynamic equilibrium in a reversible reaction?
-Dynamic equilibrium occurs when the rates of the forward and reverse reactions are equal, and the concentrations of reactants and products remain constant over time. Even though the reaction is balanced, both forward and reverse reactions continue to occur at the molecular level, but no net change in concentrations is observed.
How can equilibrium be described in terms of concentration versus time?
-At equilibrium, the concentration of reactants decreases while the concentration of products increases. Eventually, the concentrations of both reach a point where they no longer change, which is represented as horizontal lines on a graph of concentration vs. time.
What is the difference between homogeneous and heterogeneous equilibrium?
-In homogeneous equilibrium, all the reactants and products are in the same phase (e.g., gas or liquid), whereas in heterogeneous equilibrium, reactants and products are in different phases (e.g., solid, liquid, or gas). An example of homogeneous equilibrium is the reaction between gases, while heterogeneous equilibrium involves solid and gas phases, such as the dissociation of calcium carbonate.
What does the equilibrium constant (K) represent?
-The equilibrium constant (K) expresses the ratio of the concentrations or partial pressures of products to reactants at equilibrium, each raised to the power of their respective coefficients in the balanced chemical equation. This constant is temperature-dependent.
How are the equilibrium constants for concentration (Kc) and pressure (Kp) different?
-Kc applies to reactions involving concentrations (molarities) of reactants and products, while Kp applies to reactions involving the partial pressures of gases. Both constants describe the relationship between the concentrations or pressures of reactants and products at equilibrium, but Kp is specifically used when gases are involved.
What is the significance of the phases involved in a reaction when calculating the equilibrium constant?
-When calculating the equilibrium constant, only gases and aqueous species are included in the expression. Solids and liquids are not included because their concentrations do not change during the reaction and are considered constant under the given conditions.
How do you calculate the relationship between Kc and Kp?
-The relationship between Kc and Kp is given by the equation Kp = Kc * (RT)^(Δn), where R is the ideal gas constant, T is the temperature in Kelvin, and Δn is the change in the number of moles of gas (moles of products minus moles of reactants).
Why are solids and liquids not included in the equilibrium constant expression?
-Solids and liquids are excluded from the equilibrium constant expression because their concentrations remain constant throughout the reaction. Only gases and aqueous solutions are included since their concentrations can change during the reaction.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

6. Chemical Reactions (Part 3) (3/5) (Cambridge IGCSE Chemistry 0620 for 2023, 2024 & 2025)

KIMIA Kelas 11 - Kesetimbangan Kimia | GIA Academy

11 клас. Хімія. Необоротні та оборотні хімічні реакції. Хімічна рівновага. Принцип Ле Шательє

KESETIMBANGAN KIMIA KELAS 11_PART 1

Kesetimbangan Kimia| Kimia SMA | Tetty Afianti
KESETIMBANGAN KIMIA ( KIMIA SMA KELAS 11 )
5.0 / 5 (0 votes)