AQA 1.6 Equilibria REVISION
Summary
TLDRIn this educational video, Chris Harrison offers a comprehensive revision on chemical equilibria, focusing on reversible reactions and the dynamic equilibrium state where the rates of forward and reverse reactions are equal. Harrison explains Le Chatelier's principle, demonstrating how changes in pressure, temperature, and concentration affect equilibrium. He also covers the calculation of the equilibrium constant (KC), its significance, and how it's influenced by temperature but not by concentration or catalysts. The video is tailored for A-Level chemistry students, aiming to clarify complex concepts like the effect of temperature on KC and the use of ICE tables for concentration calculations at equilibrium.
Takeaways
- 🔄 Equilibria involves reversible reactions where the forward and reverse reactions occur at the same rate, leading to a dynamic equilibrium.
- 📉 At equilibrium, the concentrations of reactants and products remain constant, but the amounts can differ.
- 🔄 Le Chatelier's Principle states that if a change in pressure, temperature, or concentration is applied to a system at equilibrium, the system will adjust to counteract the change.
- 📚 The effect of concentration changes on equilibrium is observed in homogeneous equilibria, where increasing a reactant or product's concentration will shift the equilibrium to reduce that concentration.
- 📉 Increasing pressure shifts equilibrium towards the side with fewer gas particles to reduce pressure, while decreasing pressure has the opposite effect.
- 🌡️ Temperature changes affect equilibrium by shifting it in the endothermic direction when temperature is increased (for exothermic reactions), and in the exothermic direction when temperature is decreased.
- ⚖️ Catalysts speed up the rate at which equilibrium is reached but do not affect the position of equilibrium or the equilibrium constant (KC).
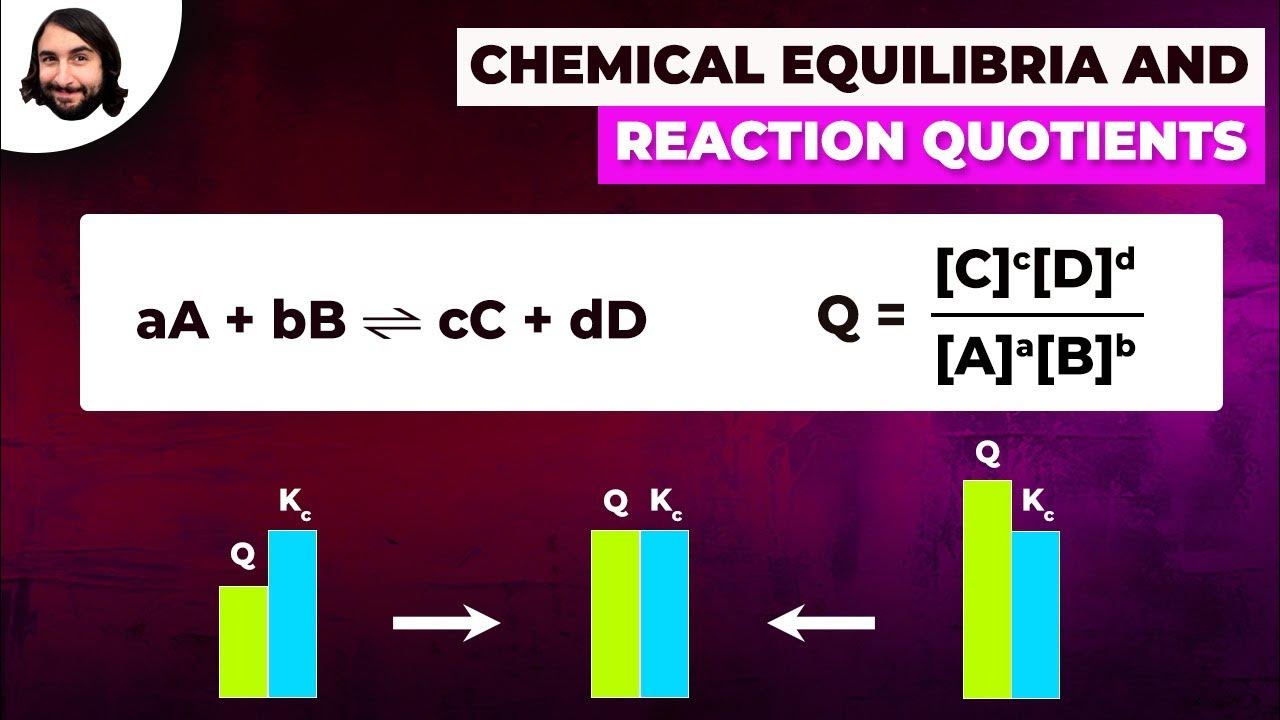
- 🔍 The equilibrium constant (KC) is calculated from the molar concentrations of reactants and products, with each substance's coefficient in the balanced equation becoming an exponent in the KC expression.
- 🧮 To find the equilibrium concentrations, you can use an ICE (Initial, Change, Equilibrium) table and the KC expression to solve for unknown concentrations.
- 🌡️ The value of KC is dependent on temperature; it increases with a decrease in temperature for exothermic reactions and decreases with an increase in temperature.
Q & A
What is the main topic of the video?
-The main topic of the video is chemical equilibria, focusing on the concept of reversible reactions and the factors that affect the position of equilibrium.
What is a dynamic equilibrium?
-A dynamic equilibrium is a state in a reversible reaction where the rate of the forward reaction equals the rate of the backward reaction, resulting in constant concentrations of reactants and products over time.
What does the video mention about the availability of the PowerPoint used in the presentation?
-The PowerPoint used in the video is available for purchase. Interested viewers can find the link in the description box of the video to access it.
According to Le Chatelier's principle, what happens when the concentration of a reactant or product is increased?
-When the concentration of a reactant or product is increased, the equilibrium will shift to try and reduce that concentration, either by consuming the added substance (if it's a reactant) or by producing more of it (if it's a product).
How does changing the pressure affect the position of equilibrium in a chemical reaction?
-Changing the pressure causes the equilibrium to shift to the side with the fewer number of gas particles, in order to counteract the change in pressure. Increasing the pressure will shift the equilibrium towards the side with fewer gas molecules, while decreasing the pressure will shift it towards the side with more gas molecules.
What is the effect of temperature changes on the position of equilibrium?
-If the temperature is increased, the equilibrium will shift in the endothermic direction to reduce the temperature, typically producing more reactants. Conversely, if the temperature is decreased, the equilibrium will shift in the exothermic direction, producing more products.
What is the role of a catalyst in a reaction at equilibrium?
-A catalyst speeds up both the forward and reverse reactions equally, thus hastening the establishment of equilibrium without affecting the position of equilibrium or the equilibrium constant (KC).
Why is it important to consider the molar concentrations and not just the amounts of reactants and products when calculating the equilibrium constant (KC)?
-The equilibrium constant (KC) is based on the molar concentrations of reactants and products, not their amounts in moles. This is because KC represents the ratio of the concentrations at equilibrium, which is necessary for comparing the extent of the reaction at equilibrium.
How does the video explain the calculation of the equilibrium constant (KC) for the reaction between SO2 and O2 to form SO3?
-The video explains that to calculate KC, one must first determine the molar concentrations of the reactants and products at equilibrium. Then, using the stoichiometry of the balanced chemical equation, the values are plugged into the KC expression, which is the product of the concentrations of the products raised to the power of their stoichiometric coefficients divided by the product of the concentrations of the reactants raised to the power of their stoichiometric coefficients.
What is the ICE table mentioned in the video, and how is it used?
-The ICE table, which stands for Initial, Change, and Equilibrium, is a method used to track the moles of reactants and products throughout a chemical reaction at equilibrium. It helps in calculating the changes in concentrations that occur as the reaction proceeds from the initial state to equilibrium.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

EQUILÍBRIO QUÍMICO: DEFINIÇÃO, CÁLCULOS E GRÁFICOS | Resumo de Química para o Enem
Chemical Equilibria and Reaction Quotients

KIMIA Kelas 11 - Kesetimbangan Kimia | GIA Academy
KESETIMBANGAN KIMIA ( KIMIA SMA KELAS 11 )

1. Equilíbrio Químico: Conceito e Características (1/2) [Físico Química]

KESETIMBANGAN KIMIA KELAS 11_PART 1
5.0 / 5 (0 votes)