05. MG2112 Termodinamika Metalurgi (Segmen 04: Kesetimbangan Es-Air, Ice Skating)
Summary
TLDRThis lecture explores thermodynamics, focusing on the phase transition of water from ice to liquid. It highlights the concept of Gibbs free energy and the Clausius-Clapeyron equation, explaining how temperature and pressure influence phase changes. Practical examples, such as ice skating and car accidents in snowy conditions, illustrate these principles. The lecture demonstrates how understanding phase transitions helps explain everyday phenomena, like why ice melts under pressure and the behavior of ice at different temperatures. It provides a real-world application of thermodynamic concepts in daily life, making complex theories accessible and relatable.
Takeaways
- 😀 The Clausius-Clapeyron equation is used to describe the relationship between pressure, temperature, and phase transitions, specifically for the example of ice and water at 1 atm and 0°C.
- 😀 At equilibrium, the Gibbs free energy (G) of both solid and liquid phases of water is minimized, meaning that the system is stable at 0°C for the given conditions.
- 😀 The change in Gibbs free energy (ΔG) during phase transitions is zero at the equilibrium point, implying no net change in free energy between the phases at this temperature.
- 😀 The transition from solid (ice) to liquid (water) occurs when ΔG = 0, which is tied to the temperature and pressure of the system.
- 😀 The Clausius-Clapeyron equation can be applied to understand the effect of pressure on the melting point of ice, where pressure increases the temperature at which ice melts.
- 😀 The phase transition of water from solid to liquid involves negative volume change (ΔV), which is unusual compared to many other substances where melting results in an increase in volume.
- 😀 The melting of ice under pressure is explained using the concept of entropy (ΔS), where pressure-induced changes in volume affect the melting process.
- 😀 Practical examples like ice skating demonstrate the impact of pressure on melting ice: the pressure from the skate blade causes a thin layer of water to form, allowing smooth movement.
- 😀 In cold climates, the Clausius-Clapeyron equation helps explain why tire pressure can cause ice to melt and lead to slippery roads, increasing the risk of accidents.
- 😀 The lecture emphasizes how understanding phase transitions and the Clausius-Clapeyron equation has practical implications in everyday life, such as in ice skating and winter driving safety.
Q & A
What is the key concept discussed in the video regarding phase transitions?
-The video primarily discusses the phase transition of water from solid (ice) to liquid, with a focus on the role of temperature, pressure, and Gibbs free energy in this process. The Clausius-Clapeyron equation is used to explain how pressure and temperature affect the melting of ice.
What role does Gibbs free energy play in the phase transition of ice to water?
-Gibbs free energy determines the spontaneity of a phase transition. At equilibrium, the Gibbs free energy of the solid and liquid phases (ice and water) are equal, indicating that the system is in a state of minimal energy. The melting of ice is spontaneous when the Gibbs free energy reaches a minimum.
What is the Clausius-Clapeyron equation, and how is it used in the video?
-The Clausius-Clapeyron equation relates the change in pressure and temperature to the phase transition, specifically the melting of ice. It describes how pressure influences the temperature at which ice melts and is essential for understanding phase changes like from solid to liquid in the context of ice and water.
How does pressure affect the melting point of ice according to the video?
-Pressure affects the melting point of ice by lowering it. When pressure is applied, such as when a skater glides over ice, the temperature at which ice melts decreases, allowing a thin layer of water to form under the ice, which reduces friction and causes the ice to melt momentarily.
What real-world example does the video give to illustrate the concept of ice melting under pressure?
-The video uses ice skating as a real-world example. When a skater applies pressure with their blades on the ice, it causes the ice to melt slightly, creating a thin layer of water beneath the blades. This reduces friction and allows the skater to glide.
What happens when ice melts at 0°C and 1 ATM pressure?
-At 0°C and 1 ATM pressure, ice melts and transitions into liquid water. This phase change occurs spontaneously because the Gibbs free energy is minimized at this temperature and pressure, and water's properties allow it to transition smoothly between solid and liquid forms.
What does the video explain about the volume change during the phase transition from solid to liquid for water?
-The video explains that water behaves uniquely during the phase transition. Unlike most substances, water expands when it freezes, but when it melts (from solid to liquid), it contracts. This change in volume is an important aspect of its behavior.
How does the video explain the role of pressure in ice skating?
-The video explains that the pressure exerted by the skate blades on the ice lowers the melting temperature of the ice, causing it to melt slightly. This creates a thin layer of water under the blades, which reduces friction and allows for smooth movement, facilitating ice skating.
What are the consequences of applying pressure to ice in everyday life, according to the video?
-Applying pressure to ice, such as from a person's weight or from car tires, causes the ice to melt at a lower temperature. This principle is relevant in situations like ice skating, where pressure from the skates causes ice to melt, and in driving on icy roads, where the risk of slipping increases when pressure is applied to the ice.
What is the significance of the volume change when ice melts in terms of practical applications?
-The volume change when ice melts is crucial for understanding how ice behaves under different conditions. In practical terms, the expansion of ice when it freezes and its contraction when it melts can affect processes like freezing water in containers, causing expansion, or creating a thin layer of water that enables smoother movement, such as in ice skating or driving on icy roads.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

KULIAH TERMODINAMIKA BAB 3 BAGIAN 1 DENGAN JUDUL SIFAT ZAT MURNI

Calculating the Energy to Melt Ice

Physics 22 Introduction to Heat & Temperature (6 of 6) Change of Phase & Latent Heat
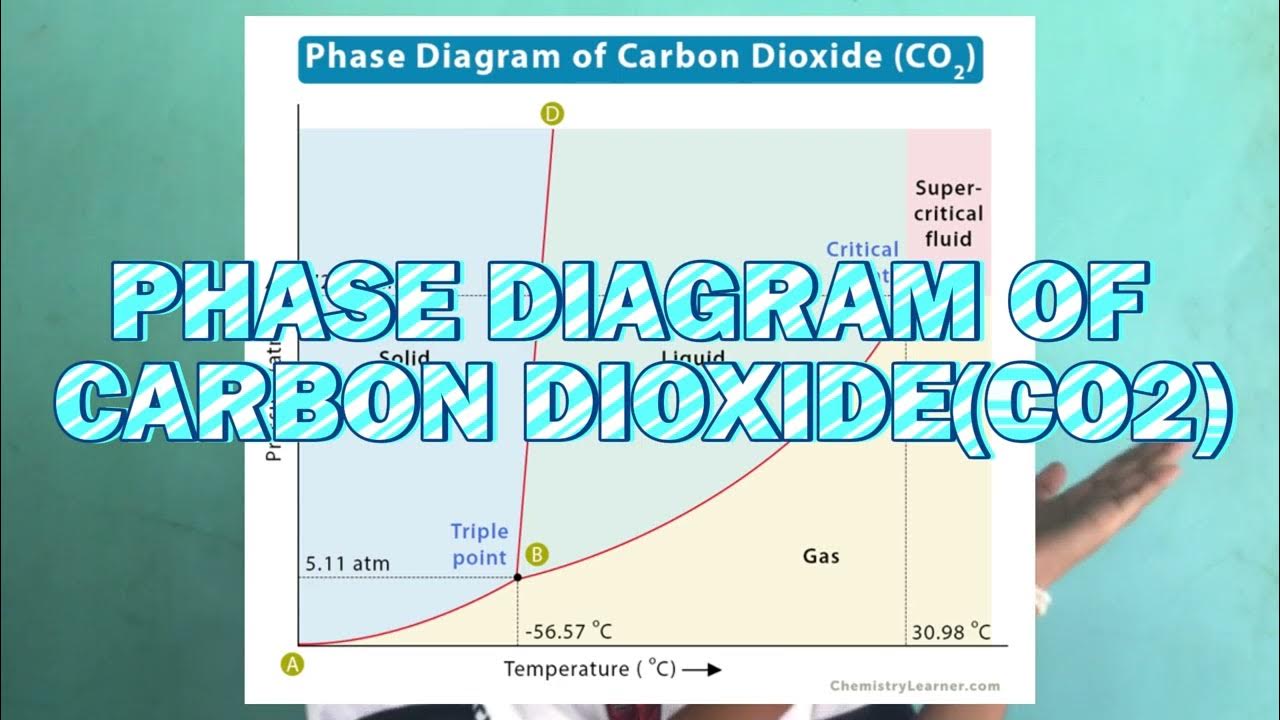
A PHASE DIAGRAM OF WATER (H2O) & CARBON DIOXIDE (CO2)

Cooling curve vs Heating curve Grade 10 Chemistry

05. MG2112 Termodinamika Metalurgi (S01: Kesetimbangan, Energi Bebas Gibbs, Potensial Kimia)
5.0 / 5 (0 votes)
