Calculating the Energy to Melt Ice
Summary
TLDRIn this instructional video, the teacher explains energy calculations related to phase changes, focusing on the concepts of heat of fusion and heat of vaporization. Using water as an example, he outlines the energy required to melt ice and transition from solid to liquid at 0 degrees Celsius. Through a detailed calculation involving 8.5 grams of ice, students learn how to apply dimensional analysis to derive the energy needed, ultimately concluding that 2,800 joules are necessary, presented in scientific notation. The lesson emphasizes the importance of significant figures and proper unit conversion in energy calculations.
Takeaways
- 🔥 Energy calculations often require understanding phase changes, not just temperature changes.
- ❄️ The formula Q = mcΔT is not applicable when there's no temperature change; ΔT equals zero.
- 💧 Heat of fusion is the energy needed to melt one gram of a substance, such as ice, at 0°C.
- ⚖️ The heat of fusion for water is 334 joules per gram.
- 💨 Heat of vaporization is the energy required to convert liquid water to gas, approximately 2260 joules per gram.
- 🔄 Temperature remains constant during phase changes, which is crucial in energy calculations.
- 📏 To find energy in phase change problems, use dimensional analysis and known values like heat of fusion or vaporization.
- 🧮 For the example of melting ice, 8.5 grams of ice requires 2800 joules of energy.
- 🔍 When performing calculations, always pay attention to significant figures to maintain accuracy.
- 🔢 Scientific notation can be used to express energy values clearly, for example, 2.8 × 10³ joules.
Q & A
What is the main concept discussed in the transcript?
-The transcript focuses on calculating energy during phase changes of substances, specifically how to account for energy when temperature remains constant.
What happens to the temperature of ice when a blowtorch is applied?
-The temperature of the ice does not change significantly while it is melting; instead, the energy is used for the phase change.
What is the formula commonly used to calculate energy changes in a substance?
-The formula is Q = mcΔT, where Q is heat energy, m is mass, c is specific heat, and ΔT is the change in temperature.
What is heat of fusion, and how is it defined?
-Heat of fusion is the energy required to melt one gram of a substance, specifically 334 joules per gram for ice to water.
What is the heat of vaporization for water?
-The heat of vaporization for water is approximately 2,260 joules per gram, used for the transition between liquid and gas.
How do you calculate the energy required to melt 8.5 grams of ice at 0°C?
-Using the formula Q = m × H_f, where m is 8.5 grams and H_f is 334 J/g, the calculation gives Q = 8.5 × 334 = 2,839 joules.
What should you consider when rounding your final answer for energy calculations?
-You should consider significant figures based on the least precise measurement involved in the calculation.
What is the final energy requirement to melt 8.5 grams of ice, considering significant figures?
-The final energy required is 2,800 joules, rounded to two significant figures, or expressed in scientific notation as 2.8 x 10³ joules.
Why is dimensional analysis important in these calculations?
-Dimensional analysis ensures that units cancel appropriately, leading to a correct final unit of energy in joules.
What key reminder does the transcript emphasize regarding phase changes?
-It emphasizes that energy remains constant when changing phases of matter, which is crucial for understanding energy calculations.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Heating Curves and Cooling Curves

Calorimetria - Aula 03 (Calor Latente)

lesson 7 4 calculating heat of phase changes

KALOR DAN PERUBAHAN WUJUD | KALOR DAN PERPINDAHAN KALOR

Physics 22 Introduction to Heat & Temperature (6 of 6) Change of Phase & Latent Heat
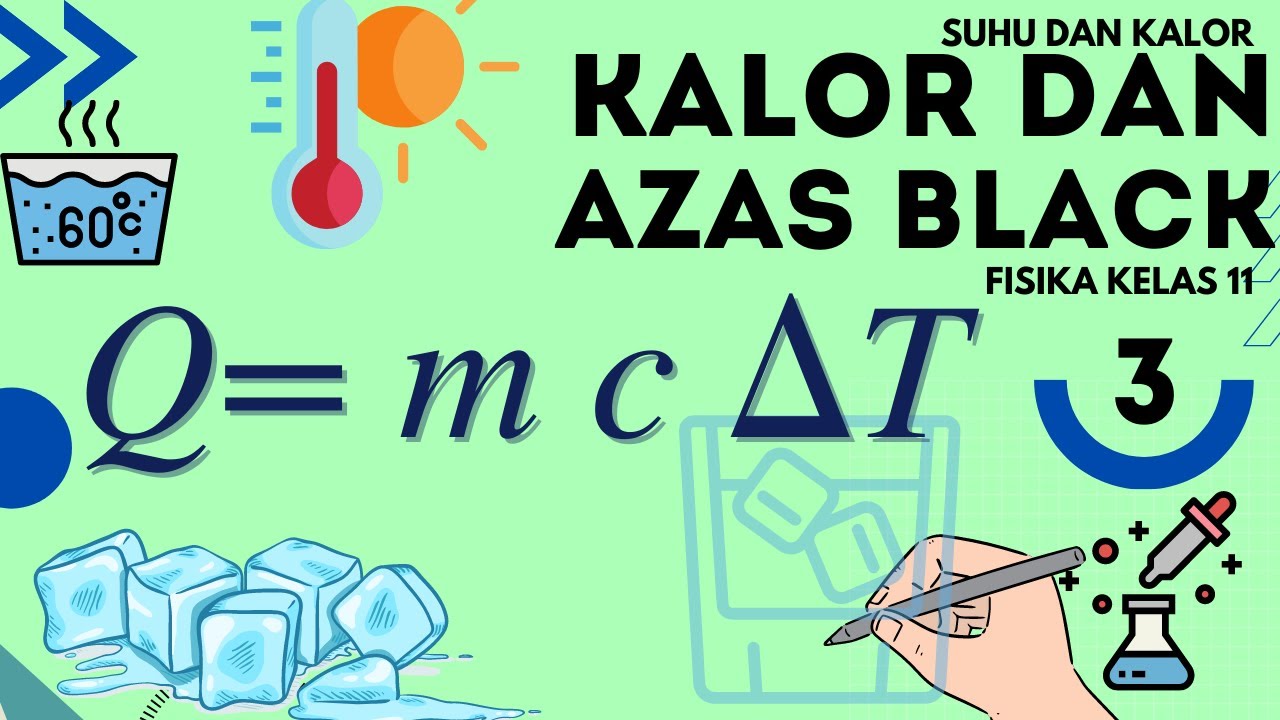
Suhu dan Kalor Fisika Kelas 11 - Part 3 : Kalor dan Azas Black
5.0 / 5 (0 votes)