PTB - Structural Bioinformatics 2
Summary
TLDRThis video explores the computational analysis of protein interactions and structural similarity. It discusses different types of protein interactions, including stable protein complexes and dynamic peptide-binding interactions. Key computational methods for identifying interaction residues and protein pockets are highlighted, along with techniques to measure structural similarity, such as RMSD calculations and sequence alignment. Emphasis is placed on the importance of structural similarity in predicting protein functions and understanding dynamic changes, with tools like Bio3D used to perform structural alignments and analyze protein interactions in the context of bioinformatics and drug design.
Takeaways
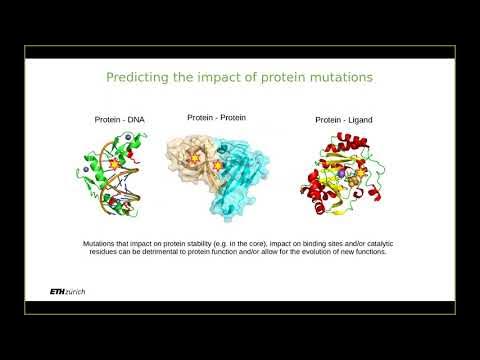
- 😀 Proteins interact in many ways, including with DNA, RNA, other proteins, and small molecules, each requiring different computational approaches for study.
- 😀 Protein-protein interactions can involve stable complexes or transient interactions, with varying degrees of binding affinity, specificity, and lifetime.
- 😀 Stable protein complexes tend to have stronger binding affinities, longer half-lives, and greater specificity, while transient interactions are often more promiscuous.
- 😀 Hydrophobic effects and ionic bonds play key roles in protein interactions, with hydrophobic residues being preferentially buried in the core or at interfaces.
- 😀 Protein pockets are distinct from protein-protein interfaces and can be studied computationally to predict small molecule binding, which is critical for drug design.
- 😀 Cryptic pockets, which are not always visible in static protein structures, may become accessible in certain conformations, adding complexity to binding predictions.
- 😀 Computational methods can identify interaction residues between proteins by calculating distances between residues or by measuring changes in surface accessibility upon binding.
- 😀 When no bound protein structure is available, predicting the protein binding state or identifying cavities for ligand docking becomes a more complex task.
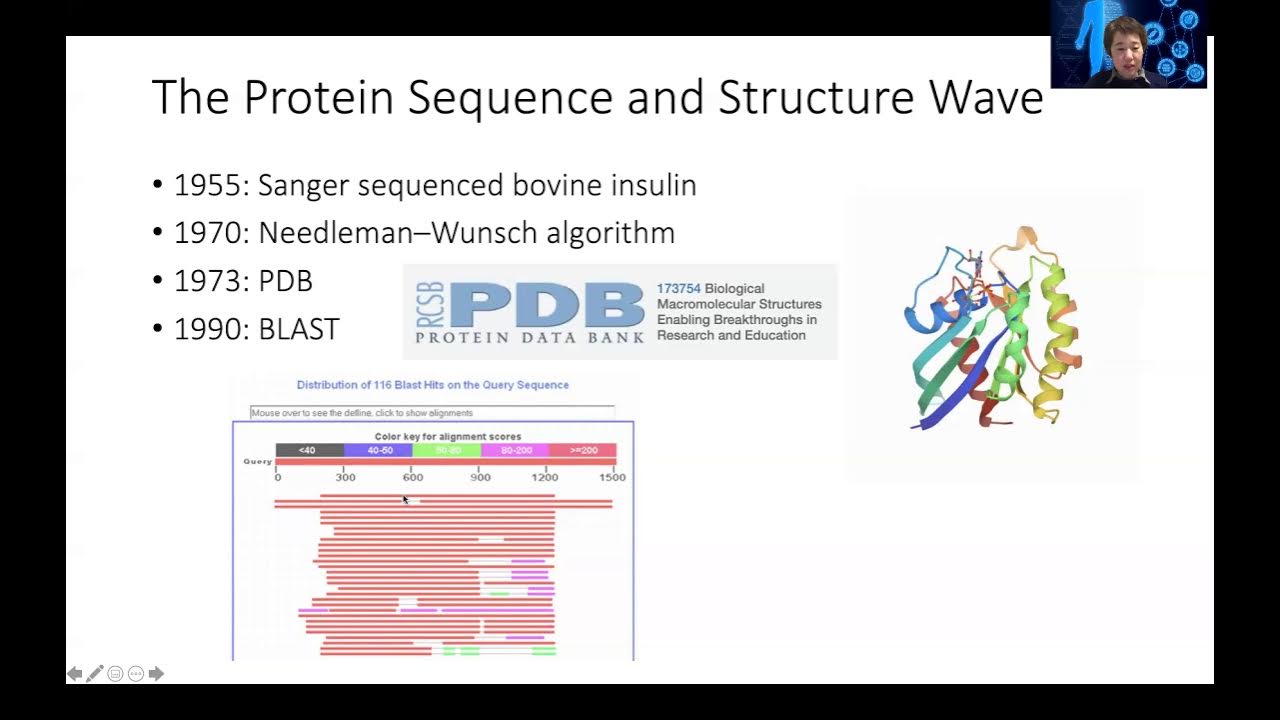
- 😀 Structural similarity, which can be more predictive of protein function than sequence similarity, is calculated by aligning structures and minimizing the RMSD between equivalent residues.
- 😀 When proteins share a sequence identity of about 30% or higher, they are likely to have similar structures, facilitating functional predictions based on structural alignment.
- 😀 Tools like Bio3D in R can be used to computationally align protein structures, calculate structural similarities, and analyze the dynamic changes of proteins, such as in the spike protein of SARS-CoV-2.
Q & A
What are the different types of protein interactions mentioned in the video?
-The video discusses several types of protein interactions, including interactions with DNA, RNA, other proteins, and small molecules. Each type of interaction has distinct properties, such as binding affinity, specificity, and duration of interaction.
What are the main differences between stable protein complexes and transient interactions?
-Stable protein complexes tend to be larger, more specific, and have stronger binding affinities with longer half-lives. In contrast, transient interactions are more dynamic, involve smaller peptide sequences, and can occur with multiple proteins, making them less specific and with weaker binding affinities.
Why is shape important in protein interactions, especially in stable complexes?
-Shape is crucial in stable protein complexes because the subunits fit together like puzzle pieces, creating highly specific interactions. This requires three-dimensional complementarity, which ensures the precise binding of proteins.
What role do hydrophobic residues play in protein interactions?
-Hydrophobic residues tend to be buried inside the protein structure or at protein interfaces, away from the surface, to minimize their exposure to the aqueous environment. This hydrophobic effect stabilizes the protein-protein interaction.
How are protein pockets involved in drug design?
-Protein pockets are crucial in drug design because small molecules can fit into these cavities, often through a 'lock and key' mechanism. Identifying and targeting these pockets, especially active or cryptic ones, allows for the development of drugs that can modulate protein activity, such as cancer therapeutics.
What is the difference between active and cryptic protein pockets?
-Active pockets are readily visible and are involved in enzymatic activity or other protein functions. Cryptic pockets are hidden under normal conditions but may become exposed when the protein undergoes conformational changes, offering new drug-targeting opportunities.
What are the two methods mentioned in the video for identifying interaction residues computationally?
-The two methods are: 1) Calculating the distance between residues in two proteins to find close pairs that likely interact. 2) Comparing the surface accessibility of residues in bound and unbound states to identify those involved in the protein-protein interface.
What is the Root Mean Square Deviation (RMSD), and how is it used in structural similarity analysis?
-RMSD is a measure of the average distance between equivalent atoms in two superimposed protein structures. It is used to calculate structural similarity by minimizing the distance between corresponding residues, where a smaller RMSD indicates greater structural similarity.
How can sequence similarity influence structural similarity in proteins?
-While sequence similarity often correlates with structural similarity, it is not always a perfect match. Even if two protein sequences are highly divergent, they may still share similar structures, especially if their functional domains are conserved.
What is the bio3D package used for in bioinformatics?
-The bio3D package in R is used for structural analysis of proteins. It allows users to align protein structures, calculate structural similarities using methods like RMSD, and analyze protein dynamics. This tool is especially useful for comparing and studying proteins computationally.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
5.0 / 5 (0 votes)