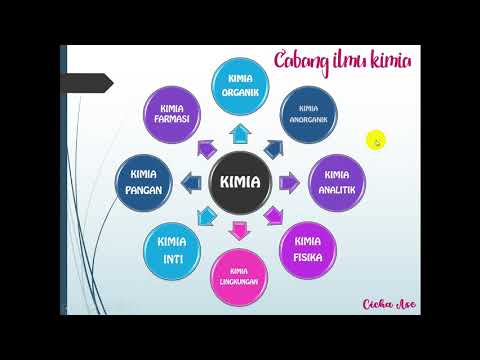
SOME DIFFERENCES IN PROPERTIES BETWEEN ORGANIC AND INORGANIC COMPOUNDS
Summary
TLDRIn this chemistry lab, students explore the differences between organic and inorganic substances through various experiments. They observe changes in color and texture upon heating substances like sugar, plastic, and aluminum foil. The combustibility of wax and table salt is tested, and solubility is demonstrated using water and kerosene with table salt and coconut oil. The lab concludes with a comparison of reaction rates between ferrous sulfate and denatured alcohol, highlighting the distinct properties of organic and inorganic compounds.
Takeaways
- 🔬 The class transitioned from inorganic to organic chemistry experiments.
- 🍬 The experiment aimed to demonstrate the differences in properties between organic and inorganic substances.
- 🔥 Heating substances like sucrose, plastic, and aluminum foil was used to observe changes in color and appearance.
- 🌾 Starch and sand were also heated to observe their reactions, including changes in color.
- 🍃 A leaf was heated to observe its organic properties upon heating.
- 🔥 The concept of combustibility was introduced, demonstrated by heating wax and table salt.
- 💧 Solubility was explored by dissolving table salt and coconut oil in water and kerosene.
- 🆚 The rates of reaction were compared between ferrous sulfate and denatured alcohol using potassium permanganate.
- ⏱️ The disappearance of potassium permanganate's color was used to compare reaction rates.
- 📝 Students were instructed to record observations and answer questions from the lab guide.
Q & A
What was the focus of the first 10 experiments performed in the class?
-The first 10 experiments were focused on inorganic chemistry.
What is the main difference between organic and inorganic chemistry as implied by the script?
-The main difference is that organic chemistry deals with carbon-containing compounds, while inorganic chemistry deals with compounds that do not contain carbon-hydrogen bonds.
What was the first substance heated in the experiment to demonstrate the composition of organic and inorganic substances?
-The first substance heated was table sugar, also known chemically as sucrose.
What happened to the table sugar when it was heated over the flame?
-The table sugar likely underwent caramelization or charring, changing its color and possibly forming a black residue.
What changes were observed in the piece of plastic when it was heated?
-The piece of plastic likely melted and possibly emitted fumes, indicating a change in its physical state upon heating.
What was the purpose of heating the aluminum foil?
-The purpose was to observe any changes in color or appearance, which could indicate its composition and reaction to heat.
How did the starch react when heated over the flame?
-The starch likely turned into a black, charred substance, indicating its organic nature and the breakdown of its molecular structure upon heating.
What was observed when sand was heated?
-There was likely no significant change in the sand's color or appearance, indicating its inorganic nature and lack of combustible elements.
What property was being tested when the wax was heated?
-The property being tested was combustibility, which refers to the ability of a substance to burn or be burned.
What happened to the table salt when it was heated?
-The table salt likely did not combust but may have changed color or melted, indicating its non-combustible nature.
What is solubility and how was it demonstrated in the experiment?
-Solubility is the ability of a substance to dissolve in a solvent. It was demonstrated by attempting to dissolve table salt and coconut oil in water and kerosene, observing their behavior in each solvent.
What was the purpose of comparing the reaction rates of ferrous sulfate and denatured alcohol?
-The purpose was to observe and compare the rates at which potassium permanganate's color disappeared when reacting with each, indicating the difference in reactivity between organic and inorganic substances.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
5.0 / 5 (0 votes)