AQA A-Level Chemistry - Ionisation Energies
Summary
TLDRThis educational video script delves into the concept of ionization energy, a crucial topic in chemistry often featured in exams. It defines ionization energy as the energy required to remove an electron from each atom in a mole of gaseous atoms to form ions. The script explains the factors influencing ionization energy, including nuclear charge, electron distance from the nucleus, and shielding effects. It also discusses trends in ionization energy across the periodic table, highlighting how these energies increase across a period and decrease down a group. Practical examples using elements like oxygen, magnesium, and sodium are provided to illustrate the principles, and the script concludes with strategies for identifying elements from their ionization energy patterns.
Takeaways
- 🔬 Ionization energy is the energy required to remove one electron from each atom in one mole of gaseous atoms to form one mole of gaseous ions.
- 📚 Memorizing definitions is crucial for scoring well on exams, as they are straightforward to understand and remember.
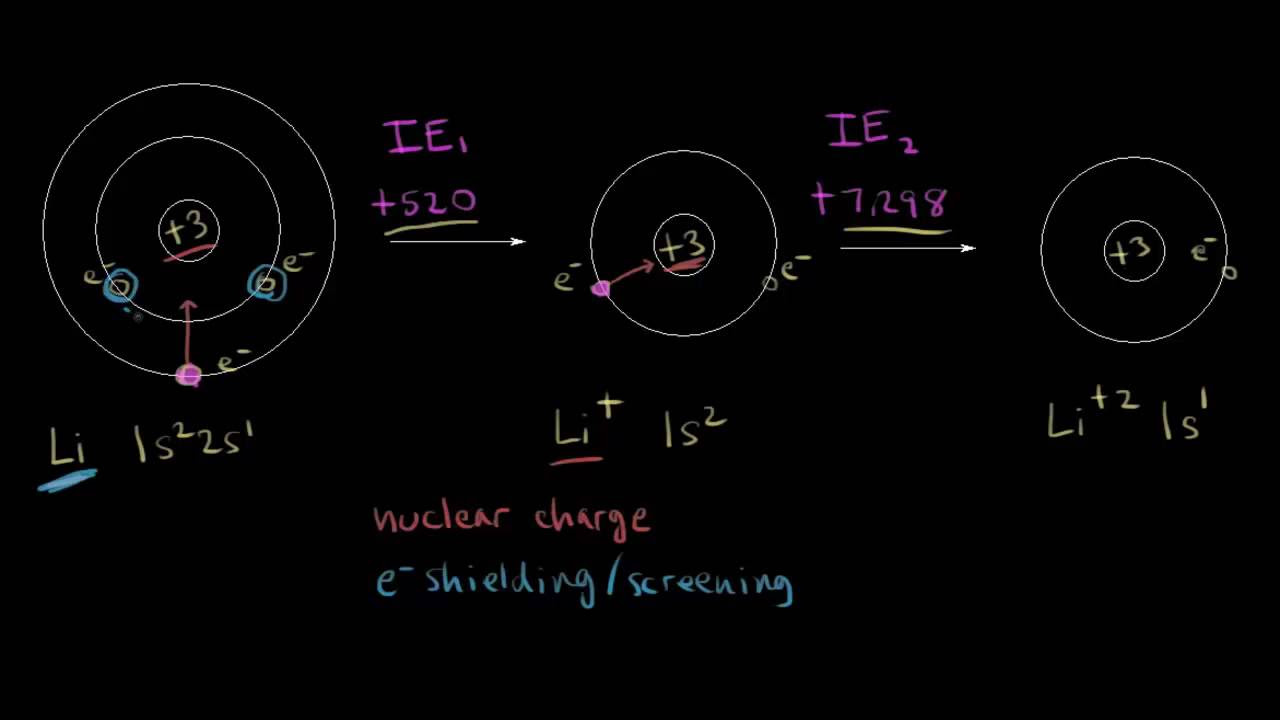
- 📈 The first ionization energy is the energy needed to remove an electron from a neutral atom, while subsequent ionization energies involve removing electrons from increasingly positive ions.
- 🔋 The number of the ionization energy asked for corresponds to the charge of the ion produced; for example, the fourth ionization energy results in a 4+ ion.
- ⚛️ Three main factors affect ionization energy: the charge of the nucleus, the distance of the electron from the nucleus, and electron shielding.
- 📉 As you move down a group in the periodic table, ionization energy generally decreases due to increasing atomic size and distance from the nucleus.
- 📈 Conversely, across a period, ionization energy increases due to decreasing atomic size and increasing nuclear charge, despite constant electron shielding.
- 📊 Graphs of ionization energy can be used to identify elements by their distinctive patterns of increases and jumps, which correspond to electron shell transitions.
- 🔑 Understanding the trends in ionization energy can help in predicting the values for consecutive ionization energies and even identifying elements from given data.
- 💡 The video provides practical exam strategies, such as using the differences in ionization energy values to estimate unknown values, which is a valuable skill for exams.
Q & A
What is ionization energy?
-Ionization energy is the energy required to remove one electron from each atom in one mole of gaseous atoms to form one mole of gaseous ions.
Why is it important to know the definition of ionization energy for exams?
-Knowing the definition of ionization energy is crucial for exams because it can be a straightforward way to earn marks, and memorizing definitions can be the difference between different grade boundaries.
What is the first ionization energy of oxygen, and how is it represented?
-The first ionization energy of oxygen involves the removal of an electron from a mole of oxygen atoms (O) to form a mole of oxygen ions (O^+) and a mole of electrons.
How does the charge of the nucleus affect ionization energy?
-The charge of the nucleus increases the attractive force between the nucleus and the outer electrons, making it more difficult to remove an electron and thus increasing the ionization energy.
What is the relationship between the distance of an electron from the nucleus and ionization energy?
-Electrons that are further from the nucleus experience less attraction and are easier to remove, resulting in a lower ionization energy compared to electrons closer to the nucleus.
How does electron shielding influence ionization energy?
-Increased shielding by inner electron shells reduces the effective nuclear charge felt by outer electrons, making them easier to remove and thus decreasing the ionization energy.
What is the significance of consecutive ionization energies in identifying elements?
-Consecutive ionization energies can be used to identify elements by recognizing patterns in the energy required to remove electrons from different energy levels.
How does the trend of ionization energy change as you move down a group in the periodic table?
-As you move down a group in the periodic table, the ionization energy generally decreases due to an increase in atomic size, distance from the nucleus, and shielding, despite an increase in nuclear charge.
What is the pattern observed in the consecutive ionization energies when moving from one energy level to another?
-There is a steady increase in ionization energy as electrons are removed from the same energy level, followed by a significant jump when moving to a lower energy level closer to the nucleus.
How can you estimate the third ionization energy of magnesium using the given data?
-By observing the trend in the consecutive ionization energies and using the difference between the first and second ionization energies, you can estimate the third ionization energy by extrapolating this trend.
Why is it easier to remove an electron from potassium compared to sodium?
-It is easier to remove an electron from potassium compared to sodium because potassium is lower in the same group of the periodic table, has a larger atomic size, and thus a lower ionization energy due to increased distance and shielding effects.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

ITB - Kuliah Online Kimia Dasar B-Latihan struktur atom dan tabel periodik-Dr. Grandprix T. M. Kadja

Sifat Keperiodikan Unsur | Kimia SMA | Tetty Afianti
First and second ionization energy | Atomic structure and properties | AP Chemistry | Khan Academy

Worked example: Identifying an element from successive ionization energies | Khan Academy

A Level Chemistry Revision "Ionisation Energy across a Period"

A Level Chemistry Revision "First Ionisation Energy"
5.0 / 5 (0 votes)
