Isotopes and Mass Spectrums
Summary
TLDRThis transcript dives into the concept of isotopes, focusing on the intriguing carbon-14 isotope and its role in carbon dating. It explains how carbon-14 is formed, its presence in living organisms, and its decay after death, which helps scientists determine the age of organic materials. The script also clarifies why atomic masses aren't always whole numbers due to the existence of multiple isotopes and their varying abundances. It further explores how a mass spectrometer detects these isotopes, providing a deeper understanding of atomic structure and isotopic analysis.
Takeaways
- 😀 Isotopes are atoms of the same element but with different masses due to varying numbers of neutrons.
- 😀 Carbon-14 is a rare isotope of carbon that is used for dating organic materials because it decays over time.
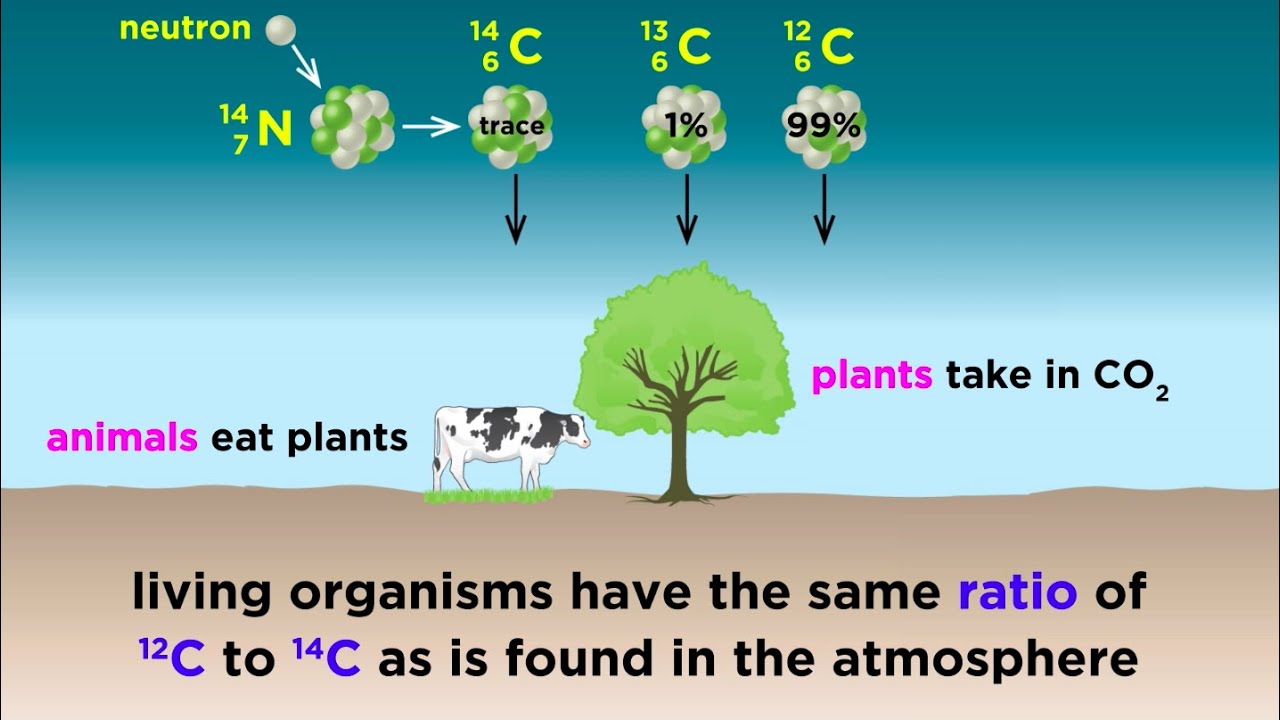
- 😀 The formation of carbon-14 happens when a neutron collides with a nitrogen-14 atom, converting it into carbon-14 and releasing a proton.
- 😀 When living organisms die, they stop absorbing carbon-14, and the amount in their bodies decreases, which is useful for determining their age (carbon dating).
- 😀 Carbon dating works for organic material up to about 50,000 years old, but it cannot be used for dating very ancient specimens, like dinosaurs.
- 😀 Atomic masses are typically not whole numbers because they are averages of the different isotopes of an element found in nature.
- 😀 For example, carbon's atomic mass is 12.011, not exactly 12, due to the presence of carbon-13 and carbon-14 isotopes in small amounts.
- 😀 The concept of half-life refers to the time it takes for half of a given amount of a radioactive isotope to decay.
- 😀 Mass spectrometers are tools used to detect and identify isotopes by measuring how atoms with different masses behave in a magnetic field.
- 😀 A mass spectrum is a chart that shows the different isotopes present in a sample, their relative abundance, and their mass-to-charge ratios.
- 😀 By analyzing the peaks in a mass spectrum, we can determine the isotopes present, their relative amounts, and even identify the element being analyzed.
Q & A
What are isotopes, and how do they differ from each other?
-Isotopes are atoms of the same element that have different numbers of neutrons. This results in different atomic masses but the same chemical properties.
How is carbon-14 formed?
-Carbon-14 is formed when a neutron collides with a nitrogen-14 atom, which causes a proton to be ejected, resulting in a carbon-14 atom.
Why is carbon-14 important for carbon dating?
-Carbon-14 is unstable and decays over time, allowing scientists to estimate the age of carbon-containing materials based on the remaining amount of carbon-14.
How does the decay of carbon-14 help in determining the age of dead organisms?
-When organisms die, they no longer take in carbon-14. As the carbon-14 in their bodies decays into nitrogen-14, the amount of carbon-14 decreases, which can be measured to estimate the time since death.
Why don't carbon-based materials from dinosaurs work for carbon dating?
-Dinosaurs lived millions of years ago, far beyond the effective range of carbon dating, which can only reliably measure objects that are up to around 50,000 years old.
What is the relationship between the mass numbers and average atomic masses on the periodic table?
-The mass number of an atom is the sum of protons and neutrons, which is usually a whole number. The average atomic mass is a weighted average of all isotopes, which results in a decimal value.
What role does a mass spectrometer play in identifying isotopes?
-A mass spectrometer separates ions based on their mass-to-charge ratio, allowing scientists to detect different isotopes of an element and determine their relative abundance.
How does a mass spectrometer work to identify isotopes?
-In a mass spectrometer, elements are ionized, accelerated, and passed through a magnetic field. The ions are bent depending on their mass, allowing the device to detect specific isotopes based on their mass-to-charge ratio.
What is a mass spectrum, and how is it used?
-A mass spectrum is a graphical representation showing the distribution of isotopes based on their mass-to-charge ratio and intensity. It helps identify the isotopes present in a sample and their relative abundances.
What can the mass spectrum of chlorine tell us about its isotopes?
-The mass spectrum of chlorine shows two isotopes, chlorine-35 and chlorine-37, with chlorine-35 being more abundant due to the higher intensity of its peak.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)