La datazione al carbonio14
Summary
TLDRThis script delves into the fascinating world of radiocarbon dating, a method used by archaeologists to determine the age of artifacts. It explains the atomic structure, focusing on carbon isotopes, particularly carbon-14. The script describes how all living organisms have carbon, and the unstable nature of carbon-14 allows for dating by measuring its decay over time. The concept of half-life is introduced, and the script touches on how the ratio of carbon-14 to carbon-12 in an organism's tissues can reveal its age upon death. It also mentions the dynamic equilibrium of carbon-14 production and decay in the atmosphere, influenced by cosmic rays and human activities, which must be considered for accurate dating.
Takeaways
- 🌿 The script discusses how archaeologists determine the age of artifacts using radiocarbon dating, specifically carbon-14.
- 🔬 Atoms are the basic building blocks of matter, composed of a nucleus with protons and neutrons, and electrons orbiting around it.
- ⚛️ Isotopes are atoms of the same element with different numbers of neutrons, like carbon-12, carbon-13, and carbon-14.
- ⏳ Carbon-14 is a radioactive isotope with a half-life of about 5,730 years, which decays into nitrogen-14.
- 🌱 Living organisms maintain a balance of carbon-14 and carbon-12 through interaction with the environment, but this balance changes upon death.
- 🕰️ The half-life of carbon-14 allows scientists to measure the time elapsed since an organism's death by comparing the remaining carbon-14 to the stable carbon-12.
- 🏺 The method of radiocarbon dating was pioneered by Willard Libby, who was awarded the Nobel Prize in Chemistry in 1960.
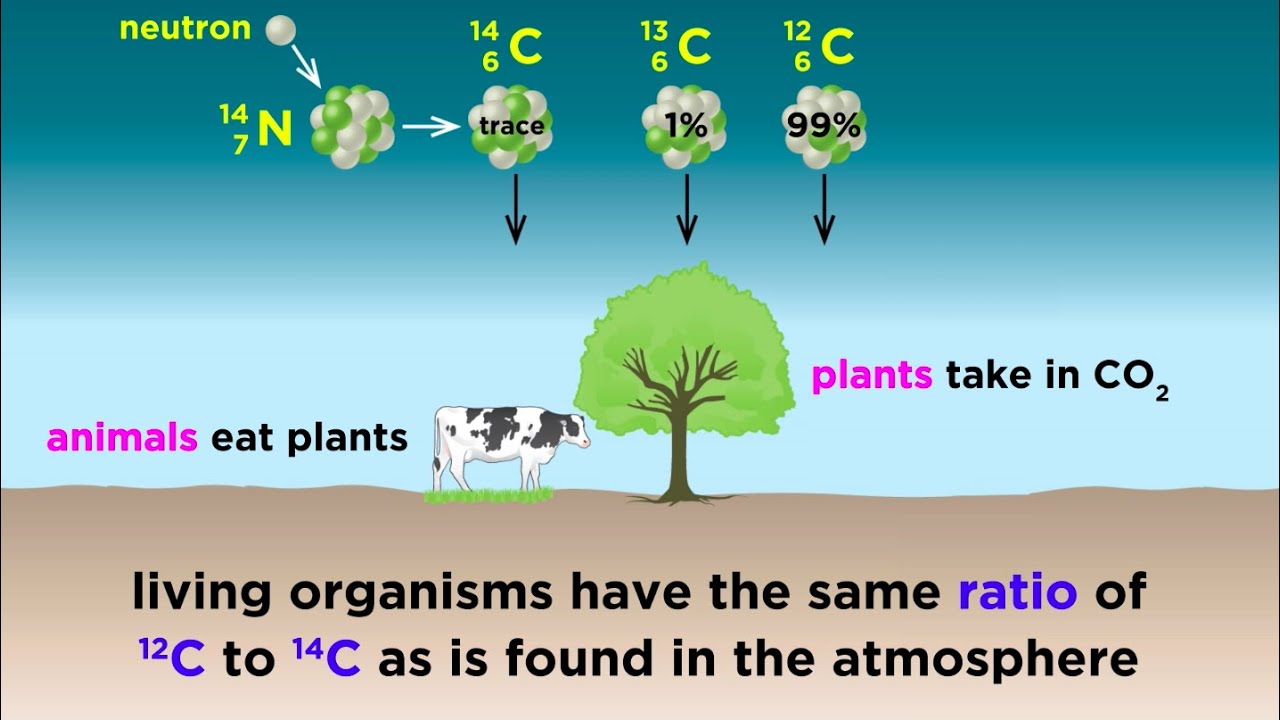
- 🌐 The carbon-14 dating method relies on the assumption that the ratio of carbon-14 to carbon-12 in the atmosphere has remained constant over time.
- 🌌 External factors such as solar activity and human activities can affect the concentration of carbon-14 in the atmosphere, impacting the accuracy of radiocarbon dating.
- 🔍 To achieve accurate dating, archaeologists must account for these variations and correct the measured carbon-14 levels accordingly.
Q & A
Why is carbon chosen for radiocarbon dating?
-Carbon is chosen for radiocarbon dating because all living organisms, including animals, plants, and humans, have carbon atoms in common. Carbon is a fundamental element in biological systems, and its isotopes can be used to determine the age of biological materials.
What is the difference between an atom and an isotope?
-An atom is the smallest unit of an element that retains the chemical properties of that element, consisting of protons, neutrons, and electrons. An isotope, on the other hand, is a variant of a particular element that has a different number of neutrons in its nucleus, resulting in a different atomic mass while retaining the same number of protons.
What is the most common isotope of carbon found in nature?
-The most common isotope of carbon found in nature is carbon-12, which makes up approximately 98.98% of all carbon atoms. It has six protons and six neutrons in its nucleus.
How does the radiocarbon dating method work?
-The radiocarbon dating method works by measuring the ratio of carbon-14 to carbon-12 in a sample. Since carbon-14 is a radioactive isotope with a known half-life of about 5,730 years, the amount of carbon-14 decreases over time in a predictable manner. By comparing the current amount of carbon-14 in a sample to the original amount, the age of the sample can be estimated.
What is the half-life of carbon-14?
-The half-life of carbon-14 is approximately 5,730 ± 30 years. This means that after 5,730 years, half of the initial carbon-14 atoms in a sample will have decayed into nitrogen-14.
Why does the carbon-14 isotope decay over time?
-Carbon-14 is an unstable isotope with an excess of neutrons in its nucleus. Over time, it decays to reach a more stable state, transforming into nitrogen-14 by emitting a beta particle.
How is the concept of 'half-life' used in radiocarbon dating?
-In radiocarbon dating, the concept of 'half-life' is used to determine the time elapsed since the organism's death. As carbon-14 decays at a known rate, the remaining amount of carbon-14 in a sample can be used to calculate its age by comparing it to the known half-life of the isotope.
What is the significance of the carbon-14 production in the atmosphere?
-The production of carbon-14 in the atmosphere is significant because it maintains a dynamic equilibrium with the decay of carbon-14 in organic materials. This continuous production ensures a relatively constant concentration of carbon-14 in the atmosphere, which is crucial for the accuracy of radiocarbon dating.
How do human activities affect the concentration of carbon-14 in the atmosphere?
-Human activities, such as the industrial revolution and nuclear testing, have affected the concentration of carbon-14 in the atmosphere. For example, the burning of fossil fuels during the industrial revolution decreased the concentration of carbon-14, while nuclear tests in the 1960s increased its production rate.
Why is it necessary to correct for historical variations in carbon-14 concentration?
-It is necessary to correct for historical variations in carbon-14 concentration to ensure the accuracy of radiocarbon dating. These variations can be caused by natural events or human activities, and they can affect the ratio of carbon-14 to carbon-12 in the atmosphere, which is used to determine the age of a sample.
Who developed the radiocarbon dating method, and what was the significance of this discovery?
-The radiocarbon dating method was developed by the American chemist Willard Libby, who was awarded the Nobel Prize in Chemistry in 1960 for his work. This discovery was significant because it provided a reliable way to date organic materials up to about 50,000 years old, revolutionizing the field of archaeology and our understanding of human history.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Carbon 14 dating 1 | Life on earth and in the universe | Cosmology & Astronomy | Khan Academy

Relative and Absolute Dating | Earth and Life Science

Bagaimana Cara Mengetahui Umur Benda Purba?
Radiometric Dating: Carbon-14 and Uranium-238

Methods of Dating the Earth Part 2: Absolute Dating (Radiometric Dating)

BAGAIMANA SEORANG ARKEOLOG BEKERJA?
5.0 / 5 (0 votes)