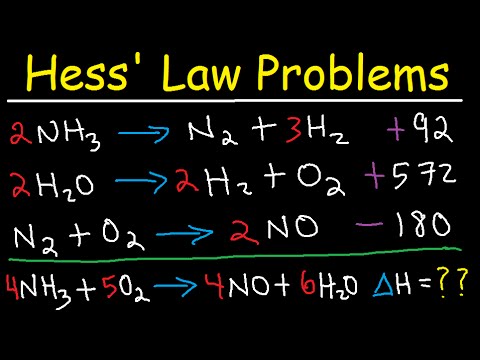
18.2 Entropy | General Chemistry
Summary
TLDRThis video focuses on understanding entropy (ΔS) in chemical reactions and processes. It highlights the importance of changes in the number of moles of gas, phase transitions, and molecular complexity in determining entropy. The script explains that an increase in the number of moles of gas and certain phase changes (like melting or dissolving) lead to a positive ΔS, while processes like freezing or condensation result in a negative ΔS. It also touches on the relationship between entropy and spontaneity, offering clear examples and encouraging a deeper understanding of the concept in chemistry.
Takeaways
- 😀 Entropy (ΔS) is a measure of disorder or randomness in a system.
- 😀 The most significant factor affecting entropy is the change in the number of moles of gas in a reaction or process.
- 😀 If the number of moles of gas increases, ΔS is positive (entropy increases).
- 😀 If the number of moles of gas decreases, ΔS is negative (entropy decreases).
- 😀 No change in the number of moles of gas makes predicting entropy based on gas alone difficult.
- 😀 Phase changes, such as melting, boiling, and sublimation, all lead to an increase in entropy (ΔS positive).
- 😀 Freezing, condensation, and deposition result in a decrease in entropy (ΔS negative) due to the transition to more ordered phases.
- 😀 Dissolving substances in water (like salts) generally increases entropy because ions are free to move in the solution.
- 😀 Complexity of molecules can affect entropy, with simpler molecules forming more complex ones potentially lowering entropy.
- 😀 When substances mix, like gases (e.g., neon and argon), the randomness increases, leading to positive entropy (ΔS > 0).
- 😀 Understanding entropy changes requires considering phase changes, gas volume changes, and molecular complexity.
- 😀 In some cases, complex biochemistry exceptions exist where dissolving substances in water may not result in a positive entropy change.
Q & A
What is entropy (ΔS), and how is it related to changes in the number of gas molecules?
-Entropy (ΔS) is a measure of the disorder or randomness in a system. When the number of gas molecules increases in a reaction, ΔS is positive because there is more randomness. Conversely, if the number of gas molecules decreases, ΔS is negative.
How do phase changes affect entropy (ΔS)?
-Phase changes play a significant role in entropy. When a substance moves from a more ordered phase to a less ordered phase (e.g., solid to liquid, liquid to gas), entropy increases (ΔS is positive). Conversely, when a substance transitions from a less ordered phase to a more ordered phase (e.g., liquid to solid, gas to liquid), entropy decreases (ΔS is negative).
What phase transitions are associated with a positive ΔS, and which with a negative ΔS?
-Positive ΔS occurs during phase changes such as melting (solid to liquid), vaporization (liquid to gas), and sublimation (solid to gas). Negative ΔS occurs during freezing (liquid to solid), condensation (gas to liquid), and deposition (gas to solid).
How does dissolving ionic compounds in water affect entropy?
-When ionic compounds like NaCl dissolve in water, the ions become free to move, which increases randomness and disorder in the system, leading to a positive change in entropy (ΔS). This is because the crystalline structure of the solid breaks apart, and the ions move independently in the solution.
Are there any exceptions to the general rule that dissolving substances in water increases entropy?
-Yes, there are exceptions. For example, some substances that do not mix well with water, like oil, do not experience a positive entropy change when they dissolve in water. These cases might involve complex interactions that do not increase disorder in the same way as salts do.
What is the primary factor to consider when determining entropy change in reactions involving gases?
-The primary factor to consider is the change in the number of moles of gas. If the number of moles of gas increases, entropy increases (positive ΔS). If the number of moles of gas decreases, entropy decreases (negative ΔS).
What is the role of molecular complexity in determining entropy change (ΔS)?
-Molecular complexity can influence entropy when there is no change in the number of gas molecules or phase changes. Generally, reactions that produce more complex molecules from simpler ones tend to have lower entropy, while simpler molecules from more complex ones increase entropy.
Why is the change in moles of gas considered the most important factor in determining entropy for many reactions?
-The change in moles of gas is the most important factor because gases have much higher entropy compared to solids and liquids. A change in the number of gas molecules leads directly to a significant change in disorder or randomness, making it a crucial factor in determining ΔS.
What happens when two gases, like neon and argon, mix together?
-When gases like neon and argon mix, the system experiences an increase in disorder because the molecules can now move freely and interact in more ways. This increase in randomness results in a positive change in entropy (ΔS).
Can entropy be predicted in reactions with no change in the number of moles of gas or phase changes?
-In reactions with no change in the number of moles of gas or phase changes, predicting entropy becomes more complex. In such cases, factors like molecular complexity may be considered, but it is often difficult to determine entropy change without further information.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)