16.2 Driving Forces of Reactions
Summary
TLDRThis video explores the driving forces behind chemical reactions, focusing on two main factors: enthalpy (ΔH) and entropy (ΔS). Enthalpy influences whether a reaction releases or absorbs energy, while entropy measures the disorder or randomness in a system. Spontaneous reactions can be driven by either a decrease in energy (exothermic reactions) or an increase in disorder (entropy-driven reactions). The concept of Gibbs Free Energy (ΔG) combines these factors to determine if a reaction is spontaneous. The video also includes a sample problem to demonstrate how Gibbs Free Energy is calculated to assess spontaneity.
Takeaways
- 😀 Reactions depend on two key factors: enthalpy (ΔH), which is the change in energy, and entropy (ΔS), which represents randomness or disorder.
- 😀 Exothermic reactions release energy and typically result in products with lower energy, making them spontaneous in nature.
- 😀 Endothermic reactions can occur if the increase in entropy outweighs the energy absorbed during the reaction.
- 😀 Entropy is a measure of randomness or disorder within a system. Higher entropy indicates a more disordered state.
- 😀 Solids have less entropy than liquids, which in turn have less entropy than gases due to the freedom of particle movement.
- 😀 At absolute zero (0K), the entropy of a perfectly ordered crystalline solid is defined as zero.
- 😀 As temperature increases, entropy also increases, especially when transitioning from solid to liquid and liquid to gas.
- 😀 The change in entropy (ΔS) during a reaction is the difference between the entropy of the products and reactants.
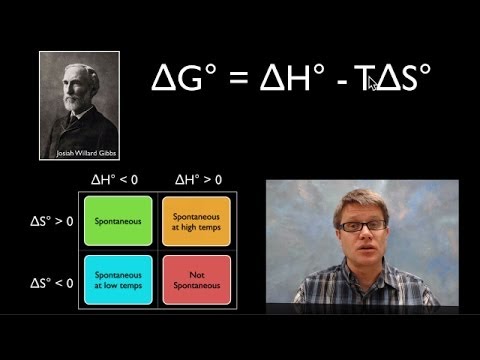
- 😀 Gibbs Free Energy (ΔG) combines enthalpy and entropy to determine whether a reaction is spontaneous: ΔG = ΔH - TΔS.
- 😀 A negative ΔG (ΔG < 0) indicates that a reaction is spontaneous, while a positive ΔG suggests it is non-spontaneous.
- 😀 In the example problem, the decomposition of ammonium chloride resulted in a positive ΔG, meaning the reaction is not spontaneous.
Q & A
What are the two primary factors that determine whether a chemical reaction will occur?
-The two primary factors are the change in energy, typically represented by enthalpy (ΔH), and the randomness or disorder in the system, represented by entropy (ΔS).
How does enthalpy (ΔH) influence a reaction?
-Enthalpy affects the energy change during a reaction. Reactions that release energy (exothermic reactions) typically occur naturally because they result in a lower energy state in the products, making it harder to reverse the process.
Can endothermic reactions occur spontaneously? If so, how?
-Yes, endothermic reactions can occur spontaneously if they lead to an increase in entropy, meaning the system becomes more disordered or random. This increase in randomness can drive the reaction, even if it absorbs heat.
What is entropy (ΔS), and why is it important in reactions?
-Entropy is a measure of the disorder or randomness in a system. It is important in reactions because an increase in entropy, such as the transition from a solid to a liquid or gas, can make a reaction spontaneous, even if it requires energy input.
How does entropy change when a solid turns into a liquid or gas?
-When a solid turns into a liquid or gas, entropy increases because the molecules in a liquid or gas are more spread out and move more freely compared to the more ordered molecules in a solid.
What is the formal definition of entropy at absolute zero (0 K)?
-At absolute zero (0 K), entropy is defined as zero for a perfectly ordered crystalline solid. However, this state is unattainable in practice.
How is entropy related to the physical state of matter?
-Solids have lower entropy than liquids, and liquids have lower entropy than gases because the particles in solids are more tightly bound in an orderly fashion, while particles in liquids and gases are more randomly arranged and free to move.
What is the formula for Gibbs Free Energy (ΔG), and how is it used?
-The formula for Gibbs Free Energy is ΔG = ΔH - TΔS, where ΔH is the change in enthalpy, T is the temperature in Kelvin, and ΔS is the change in entropy. It is used to determine if a reaction is spontaneous. If ΔG is negative, the reaction is spontaneous.
What does it mean if the Gibbs Free Energy (ΔG) of a reaction is positive?
-If ΔG is positive, the reaction is not spontaneous, meaning it cannot occur naturally under the given conditions.
In the example of ammonium chloride decomposition, why was the reaction not spontaneous?
-The reaction of ammonium chloride decomposition was not spontaneous because the calculated Gibbs Free Energy (ΔG) was positive (+91 kJ/mol), meaning the reaction requires more energy than it releases and cannot occur spontaneously.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Gibbs Free Energy
Using Gibbs Free Energy
Thermodynamics and Energy Diagrams: Crash Course Organic Chemistry #15

ATURAN DALAM PERHITUNGAN PERUBAHAN ENTALPI

Termokimia (1) | Entalpi Dan Perubahan Entalpi | Persamaan Termokimia | Hukum Hess

Energy and Chemical Change grade 11: Activation energy, heat of reaction, catalyst and MORE!
5.0 / 5 (0 votes)