Fisika Kelas 11 | Termodinamika
Summary
TLDRThis video provides a detailed overview of thermodynamics, starting with the first law, which focuses on the conservation of energy and the relationship between heat (Q) and work (W). It then covers different thermodynamic processes, including isobaric, isochoric, isotermal, and adiabatic processes, with explanations on how energy changes in each case. The video also delves into the second law of thermodynamics, entropy, and introduces concepts like the Carnot engine and refrigeration systems. It offers practical examples and mathematical formulas, helping viewers understand the theory and its real-world applications in machines like engines and refrigerators.
Takeaways
- 😀 Thermodynamics is the branch of physics that deals with the laws governing heat and work, including energy transfer and transformations.
- 😀 The First Law of Thermodynamics is essentially the law of energy conservation: energy cannot be created or destroyed, only transformed from one form to another.
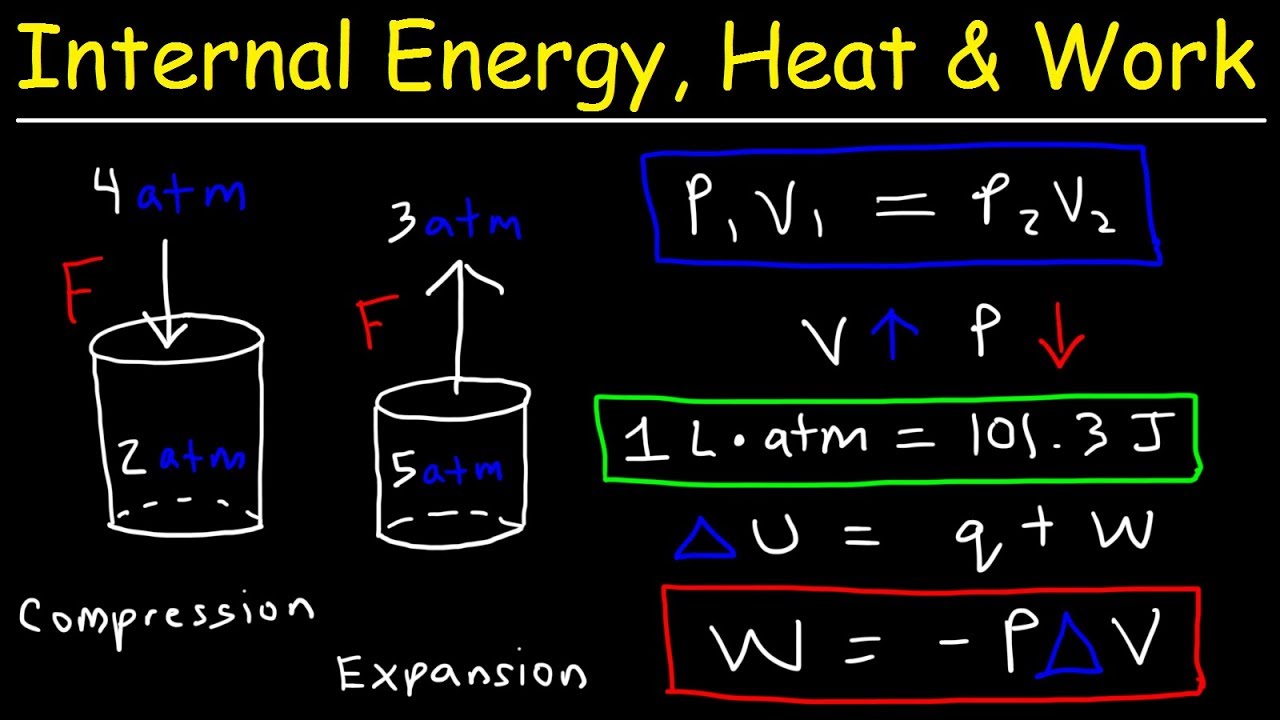
- 😀 The formula for the First Law is ΔU = Q - W, where ΔU is the change in internal energy, Q is heat added to the system, and W is the work done by the system.
- 😀 Heat can be transferred to a system (positive Q) or released by a system (negative Q), and work can be done by the system (positive W) or on the system (negative W).
- 😀 The behavior of gases in thermodynamic processes is described by the ideal gas law and equations such as P1V1/T1 = P2V2/T2 for different conditions.
- 😀 Thermodynamic processes can be categorized into isobaric (constant pressure), isochoric (constant volume), isotermal (constant temperature), and adiabatic (no heat exchange) processes.
- 😀 In an isobaric process, the pressure remains constant while volume changes. The work done is calculated as W = P * ΔV.
- 😀 For an isochoric process, the volume is constant, and thus no work is done (W = 0), but energy changes still occur based on heat exchange.
- 😀 In an isotermal process, the temperature remains constant, and the work done by the system is related to the heat added through the equation W = nRT ln(V2/V1).
- 😀 An adiabatic process involves no heat exchange with the surroundings, and the system does work without heat transfer, often characterized by the relation P1V1^γ = P2V2^γ.
- 😀 The Second Law of Thermodynamics states that heat flows from a higher temperature to a lower one spontaneously, and it introduces the concept of entropy. In irreversible processes, entropy increases.
- 😀 The Carnot engine operates between two temperature reservoirs and is a theoretical ideal heat engine that operates in two isotermal and two adiabatic processes. Its efficiency depends on the temperatures of the reservoirs.
- 😀 Refrigerators and air conditioners work by transferring heat from a cold reservoir to a hot one, requiring work to reverse the natural flow of heat. Their performance is measured by the coefficient of performance (COP).
Q & A
What is the main focus of thermodynamics as a field of study?
-Thermodynamics is the branch of physics that studies the laws governing heat, work, and energy transfer. It primarily focuses on understanding how energy, in the form of heat, moves and transforms between systems.
What is the First Law of Thermodynamics, and what does it state?
-The First Law of Thermodynamics is essentially the law of conservation of energy, stating that energy cannot be created or destroyed, only transformed from one form to another. In mathematical form, this is expressed as ΔU = Q - W, where ΔU is the change in internal energy, Q is heat added to the system, and W is the work done by the system.
What are the key variables involved in the First Law of Thermodynamics and their meanings?
-In the First Law of Thermodynamics, the key variables are ΔU (the change in internal energy, measured in Joules), Q (the heat added to or removed from the system, positive for heat added and negative for heat removed), and W (the work done by or on the system, positive when the system does work and negative when the system receives work).
What are the four main types of thermodynamic processes?
-The four main thermodynamic processes are: isobaric (constant pressure), isochoric (constant volume), isotermal (constant temperature), and adiabatic (no heat exchange). Each process follows specific equations that describe how variables like pressure, volume, and temperature interact.
How does the work equation change for each thermodynamic process?
-For an isobaric process, the work done is W = P * ΔV (constant pressure). For an isochoric process, no work is done (W = 0) because the volume does not change. For an isotermal process, the work equation is W = nRT ln(V2/V1). In an adiabatic process, work can be calculated using W = (P1V1 - P2V2) / (γ - 1), where γ is the adiabatic index.
What is the difference between the First and Second Laws of Thermodynamics?
-The First Law deals with the conservation of energy, stating that energy cannot be created or destroyed but can change forms. The Second Law, on the other hand, introduces the concept of entropy, stating that the total entropy of an isolated system always increases over time in irreversible processes, and that heat naturally flows from high to low temperatures.
What is entropy, and how is it related to the Second Law of Thermodynamics?
-Entropy is a measure of the disorder or randomness in a system. According to the Second Law of Thermodynamics, the total entropy of an isolated system tends to increase over time, especially during irreversible processes. In reversible processes, the change in entropy is zero.
How does a Carnot engine work, and what is its efficiency formula?
-A Carnot engine is an idealized heat engine that operates between two heat reservoirs, one at high temperature (T1) and one at low temperature (T2). It undergoes two isotermal and two adiabatic processes. The efficiency of a Carnot engine is given by the formula: η = 1 - (T2 / T1), where T1 and T2 are the absolute temperatures of the hot and cold reservoirs, respectively.
What is the role of a refrigeration cycle in thermodynamics, and how does it differ from a Carnot engine?
-A refrigeration cycle is the reverse process of a Carnot engine. It moves heat from a low-temperature reservoir to a high-temperature reservoir by performing work. Unlike a Carnot engine, which produces work from heat, a refrigeration cycle requires work input to transfer heat against its natural flow (from cold to hot). The coefficient of performance (COP) for a refrigeration cycle is given by COP = Q2 / W, where Q2 is the heat removed and W is the work input.
How do the equations of state relate to the First Law of Thermodynamics?
-The equations of state, such as the ideal gas law (PV = nRT), provide relationships between pressure, volume, and temperature for a given system. These equations help in understanding how the thermodynamic properties of a system change during processes like isobaric, isochoric, and isotermal, and they are often used to calculate changes in internal energy, work, and heat according to the First Law of Thermodynamics.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

BTEC Applied Science: Unit 5 Physics The First Law of Thermodynamics

First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry

Materi Fisika Kelas 11 Termodinamika
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume, Chemistry Problems

Panas Ch. Hukum I Termodinamika - Pembimbing Akademik

1ª LEI DA TERMODINÂMICA | Resumo de Física para o Enem
5.0 / 5 (0 votes)
