Ice Melting in Water: Does the Water Level Change?
Summary
TLDRIn this video, the concept of buoyancy is explored through a classic physics question: What happens to the water level in a glass when a chunk of ice melts? The discussion delves into the physics behind the buoyant force, the forces acting on the ice, and how the volume of displaced water relates to the mass of the ice. The video explains that as the ice melts, it becomes water that fills the space it previously occupied, keeping the water level the same. Through a mix of humor and detailed explanations, the video demonstrates how the principles of physics work in real-life scenarios.
Takeaways
- 😀 The script explores a classic buoyancy problem involving a chunk of ice floating in a glass of water and whether the water level changes as the ice melts.
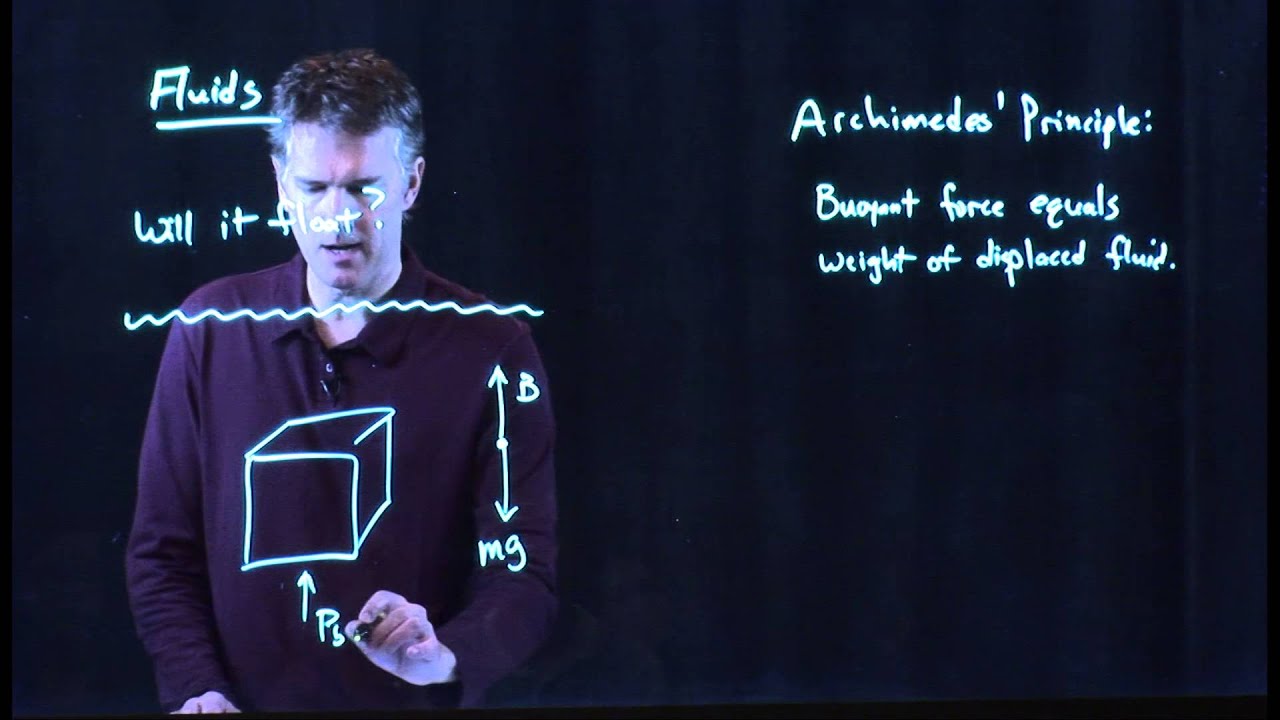
- 😀 The key principle discussed is the buoyant force, which acts upwards on the ice, while the force of gravity pulls it down.
- 😀 The ice floats because its density is less than that of water, leading to a force balance between the buoyant force and gravitational force.
- 😀 The forces acting on the ice chunk are analyzed in terms of the free body diagram, where the net force in the y-direction is zero.
- 😀 The net force equation (buoyant force = force of gravity) is used to show that the mass of the fluid displaced by the ice equals the mass of the ice chunk.
- 😀 The relationship between mass, volume, and density is introduced, with the equation density = mass/volume.
- 😀 By substituting mass with density times volume, the script demonstrates that the mass of displaced fluid equals the mass of the ice.
- 😀 The video suggests a thought experiment: removing the ice and replacing it with an equal mass of water to see how the water level is affected.
- 😀 As the ice melts, it turns into water and occupies the same volume of space below the waterline, meaning the water level does not change.
- 😀 The script emphasizes the role of density change during the phase transition from ice to water, where water's density increases, but the volume occupied stays the same.
- 😀 The characters express their satisfaction as they confirm that the physics behind the problem works out as expected, with the water level remaining constant.
Q & A
Why does ice float in water?
-Ice floats in water because its density is less than that of water, causing it to be buoyant.
What happens to the water level as the ice melts?
-The water level remains the same as the ice melts because the volume of water displaced by the ice is equal to the volume of the water that replaces it as the ice melts.
What is the first step in solving the buoyancy problem in the script?
-The first step is to draw a free body diagram of the forces acting on the chunk of ice, including the buoyant force and the force of gravity.
What forces are acting on the chunk of ice?
-The two main forces acting on the chunk of ice are the buoyant force, which is directed upwards, and the force of gravity, which is directed downwards.
How do the forces on the ice chunk result in it floating?
-The buoyant force equals the force of gravity, which keeps the ice chunk floating without accelerating, thus maintaining equilibrium.
What equation is used to relate mass and density?
-The equation used is mass equals density times volume (mass = density × volume).
What happens when the chunk of ice is replaced by water?
-When the ice is replaced by water, the water fills the space below the waterline that was previously occupied by the ice, maintaining the same water level.
Why does the density of the substance increase as ice melts into water?
-The density of the substance increases as ice melts into water because the molecules in water are more tightly packed than in ice.
What did the video show in relation to the ice melting?
-The video demonstrated that as the ice melts, the water level stays the same, confirming the theoretical explanation.
What did the speaker emphasize about the physics of the situation?
-The speaker emphasized that the physics works, and the water level stays the same due to the principle of displacement and density changes as ice melts.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)