KIMIA ANALISIS GRAVIMETRI (PART 1)
Summary
TLDRThis video provides an informative explanation of gravimetric analysis techniques. It covers six key methods: evaporation, ashing, electrolysis, extraction, distillation, and precipitation. The video discusses the principles behind each method, such as mass reduction, temperature control, and sample separation, and highlights their applications in determining components like water content, ash, metals, and minerals. Aimed at helping viewers understand these techniques, the video emphasizes their importance in chemical analysis, with a focus on precise measurements and separation methods for various substances.
Takeaways
- 😀 Gravimetric analysis is a quantitative method based on measuring the mass of an analyte.
- 😀 There are several types of gravimetric analysis techniques: evaporation, ashing, electrolysis, extraction, distillation, and precipitation.
- 😀 Evaporation technique reduces the mass of components in a sample by evaporating it at a specific temperature, typically used to determine moisture content in samples.
- 😀 Ashing involves heating the sample at high temperatures (up to 550°C) to determine the ash content, dry matter, total minerals, and other total solids.
- 😀 Electrolysis technique relies on the deposition of a sample onto one of the electrodes during electrolysis, commonly used for analyzing metals in liquid mixtures.
- 😀 Extraction is the process of separating the analyte from a sample through extraction stages, often used for determining metals, fats, minerals, or natural substances.
- 😀 Distillation separates the analyte by heating the sample, commonly used to determine the water content in substances or extract essential oils.
- 😀 Precipitation is the most commonly used gravimetric method, where the analyte forms an insoluble compound in solution, often used to determine phosphorus, sulfur, calcium, or nickel content.
- 😀 The evaporation method is ideal for determining water content in solids, suspended solids, dissolved solids, and volatile components.
- 😀 The video encourages viewers to subscribe, like, and comment for more detailed explanations in the next video.
Q & A
What is gravimetric analysis?
-Gravimetric analysis is a quantitative analysis method based on the measurement of the mass of an analyte.
What are the main types of gravimetric analysis techniques?
-The main types of gravimetric analysis techniques include evaporation, ashing, electrolysis, extraction, distillation, and precipitation.
What is the principle behind the evaporation technique in gravimetric analysis?
-The evaporation technique is based on the reduction of mass of components in the sample by evaporating it at a specific temperature. It is typically used to determine water content in a sample.
How does ashing differ from evaporation in gravimetric analysis?
-While evaporation involves reducing the mass of components by evaporating at a controlled temperature, ashing is done at much higher temperatures, up to 550°C, to determine the ash content, mineral content, or total solids in the sample.
What is the role of electrolysis in gravimetric analysis?
-In electrolysis, the analyte is deposited on one of the electrodes during an electrolysis process. This technique is commonly used for analyzing metals in mixtures, mineral purity, and waste content.
What does the extraction technique in gravimetric analysis involve?
-Extraction is a method used to separate an analyte from a sample through an extraction process. It is often applied to determine metals, fats, minerals, or natural extracts.
What is distillation and how is it used in gravimetric analysis?
-Distillation is a technique where the analyte is separated through a distillation process. It is commonly used to determine the water content in materials and extract essential oils.
What is the most widely used technique in gravimetric analysis?
-Precipitation is the most widely used technique in gravimetric analysis. It involves the formation of an insoluble precipitate in a solution, often used for determining elements like phosphorus, sulfur, calcium, and nickel.
What type of substances are commonly analyzed using gravimetric analysis?
-Gravimetric analysis is commonly used to analyze substances such as metals, minerals, water content, ash content, and various chemicals in solid and liquid samples.
Why is gravimetric analysis considered a reliable method?
-Gravimetric analysis is considered reliable because it directly measures the mass of the analyte, providing highly accurate and precise results, especially when the sample preparation and measurement steps are carefully controlled.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

ANALISIS GRAVIMETRI (PART 2) Perhitungan Gravimetri Metode Pengendapan

Gravimetric Analysis Video

GRAVIMETRY ANALYSIS METHOD - PART 1

Praktikum Kimia Analisis - 8. Gravimetri (Bagian Kedua)
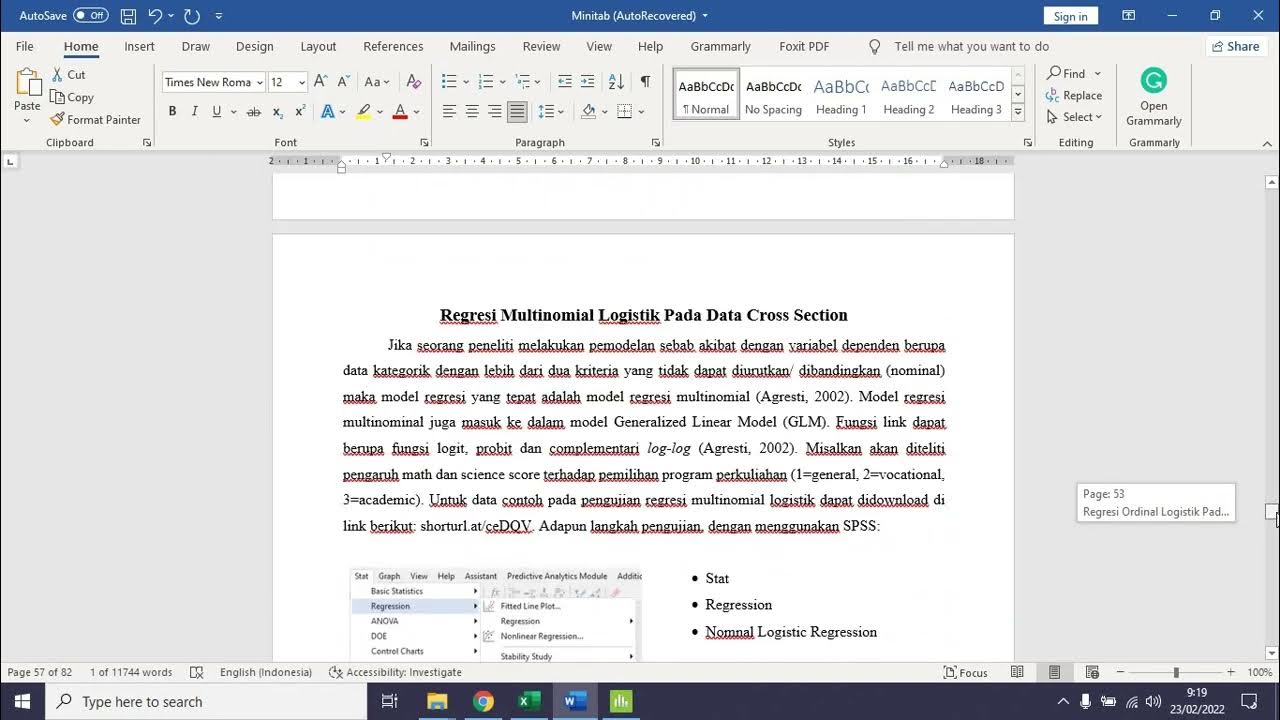
Regresi Ordinal dan Multinomial Logistik Pada Data Crosssection dengan Minitab

[FSH SPECIAL TOPICS] Types of Gravimetry - Analytical Chemistry
5.0 / 5 (0 votes)
