GRAVIMETRY ANALYSIS METHOD - PART 1
Summary
TLDRThis video tutorial provides a comprehensive overview of gravimetric analysis, a quantitative analytical method focused on the purification and weighing of substances. It explains three main techniques: precipitation, evaporation, and electrolysis, detailing the principles and necessary conditions for each method. The tutorial emphasizes the importance of accurate measurements and the role of various reagents and equipment. By the end, viewers are encouraged to apply these techniques in laboratory settings and are invited to learn more about the calculation processes in subsequent videos.
Takeaways
- 🔍 Gravimetric analysis is a quantitative method based on measuring the mass of a specific component in a sample.
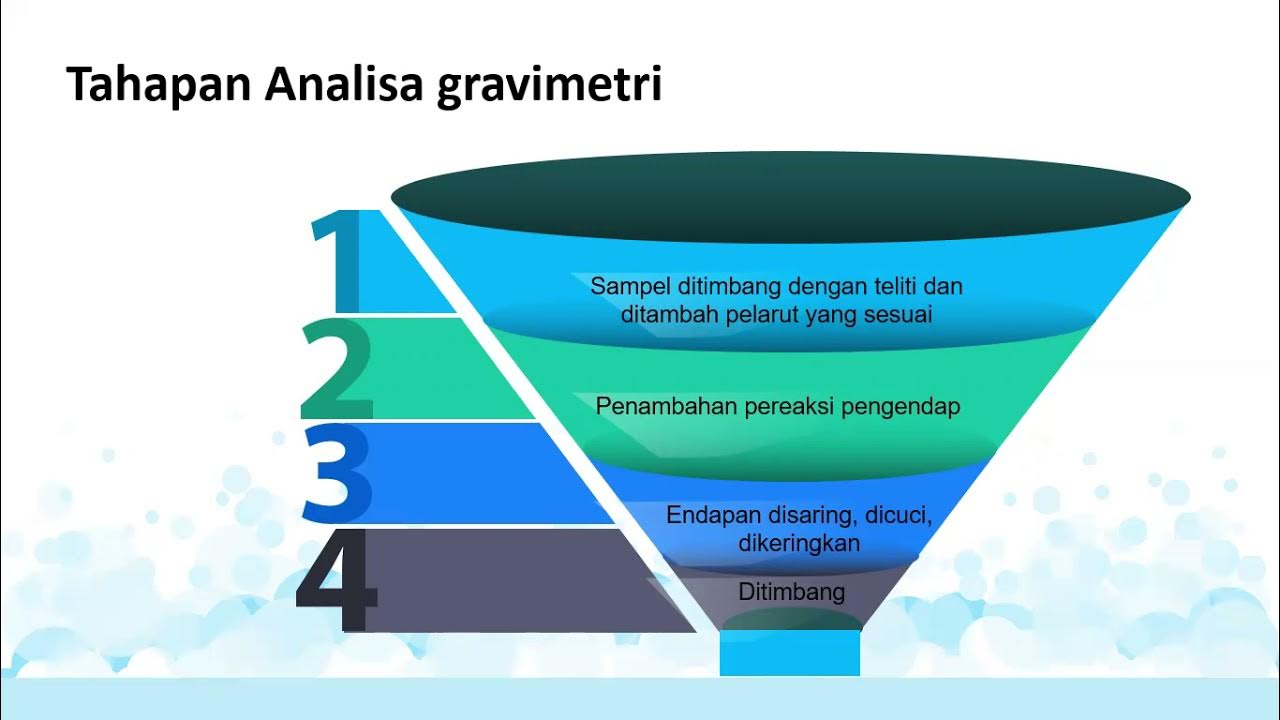
- ⚗️ The process involves several steps: precipitation, filtration, drying, and weighing the precipitate.
- 📏 Essential equipment includes analytical balances, ovens for drying, desiccators for moisture control, and crucibles for ashing.
- 💧 The precipitation method involves adding a reagent to form an insoluble compound from the target analyte.
- 🌡️ Conditions such as pH and temperature significantly affect the efficiency of precipitation.
- 🔥 The evaporation method determines the mass of volatile components, especially in hydrated compounds.
- ⚡ Electrolysis reduces metal ions in solution to solid metal deposits on electrodes, allowing for concentration measurement.
- 🔗 It is crucial to ensure that samples are dilute for effective separation of precipitates.
- 🧪 Maintaining optimal pH is necessary for successful precipitation of desired compounds.
- 📊 Future discussions will include detailed calculations related to gravimetric analysis.
Q & A
What is gravimetric analysis?
-Gravimetric analysis is a quantitative analytical technique that determines the amount of an analyte based on the mass of a solid.
What are the main steps involved in gravimetric analysis?
-The main steps include sample preparation, precipitation of the analyte, filtering and washing the precipitate, drying or incinerating the precipitate, and weighing it for analysis.
What types of methods are commonly used in gravimetric analysis?
-Common methods include precipitation, evaporation, and electrolysis.
How does the precipitation method work in gravimetric analysis?
-In the precipitation method, a reagent is added to the sample to form an insoluble precipitate, which is then collected, washed, and weighed to determine the analyte's mass.
What role does drying or incineration play in gravimetric analysis?
-Drying or incineration is crucial for removing moisture or impurities from the precipitate, ensuring accurate weight measurements for analysis.
What is the purpose of filtering the precipitate?
-Filtering the precipitate separates it from the solution and removes any unreacted reagents or impurities that could affect the mass measurement.
What is the significance of stoichiometry in gravimetric analysis?
-Stoichiometry is important because it allows for the calculation of the concentration of the analyte based on the mass of the precipitate and the chemical reactions involved.
Why is the electrolysis method mentioned in the context of gravimetric analysis?
-The electrolysis method is discussed as a way to reduce metal ions to solid metal, allowing for mass measurement, thus providing a quantitative assessment of the analyte.
What factors can affect the accuracy of gravimetric analysis?
-Factors include the purity of reagents, precision in weighing, completeness of precipitation, and proper drying or incineration of the precipitate.
How can gravimetric analysis be applied in real-world scenarios?
-Gravimetric analysis can be used in various fields such as environmental testing, food analysis, and pharmaceuticals to determine concentrations of substances in different samples.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)