Kalor - Grafik Perubahan Wujud Zat (Fisika)
Summary
TLDRIn this educational video, the process of calculating the amount of heat required for phase changes in water is discussed. The example problem involves heating 250 grams of ice from -5°C to 0°C, causing it to melt, and how to calculate the heat for each stage: warming, melting, and phase transitions. The video explains the specific heat capacities and latent heat involved, and uses clear formulas for each part of the process. Additionally, a second example is given with a 200-gram ice sample heated to 100°C. The explanations are illustrated through graphical representations of phase changes and calculations.
Takeaways
- 😀 The video explains the concept of heat transfer and how it affects the phase changes of matter, specifically water.
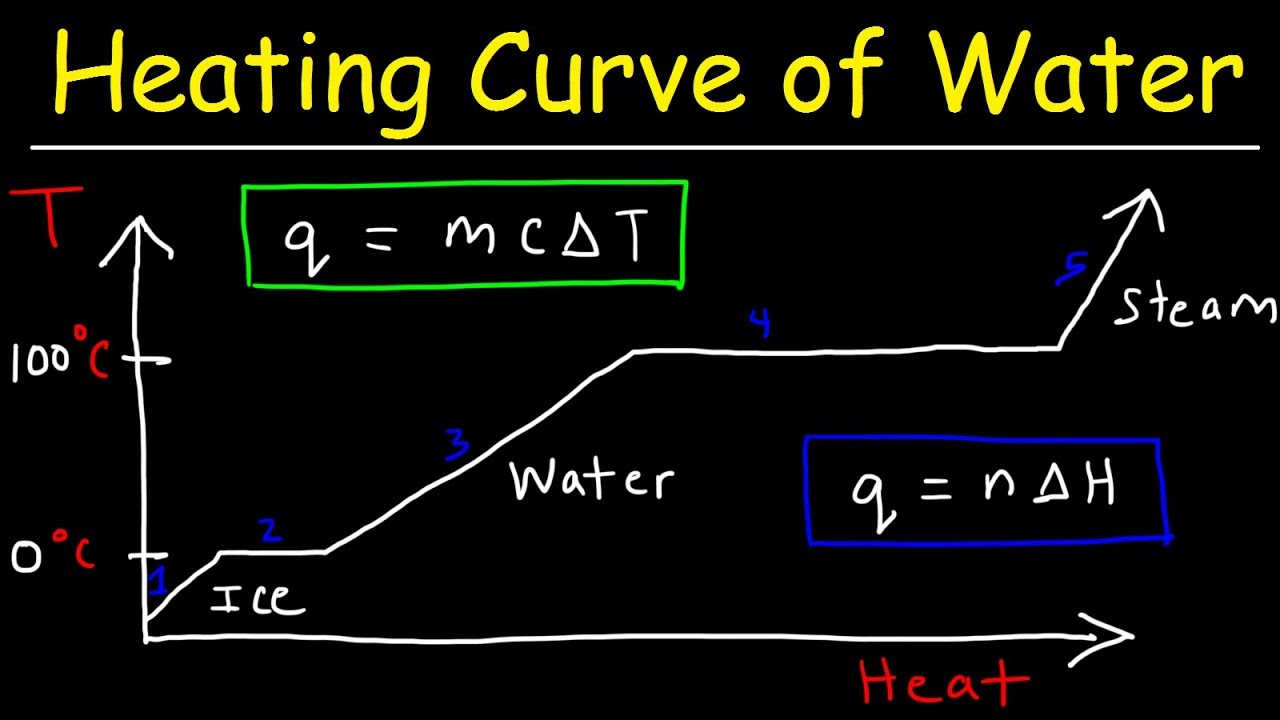
- 😀 Previous videos covered the basic formula for heat calculation: Q = m * c * ΔT, which is used to calculate the amount of heat required to change the temperature of a substance.
- 😀 A practical example involves heating 250 grams of ice at -5°C until it melts to become water at 0°C. The required heat is calculated using different formulas for each stage of the process.
- 😀 The video introduces a graph showing the phase changes of water: solid (ice) to liquid (water), and liquid to gas (vapor), with distinct stages requiring different calculations.
- 😀 For the process of heating, the formula Q = m * c * ΔT is used when the substance’s temperature changes without a phase change.
- 😀 During phase changes, such as melting or vaporization, the formulas change: Q = m * l (for melting) and Q = m * u (for vaporization), where l and u represent the latent heat of fusion and vaporization, respectively.
- 😀 The script explains how to apply the formulas step by step, starting with the increase in temperature (Q1) and then the phase change (Q2).
- 😀 For example, to calculate the heat needed to melt ice at -5°C and raise it to 0°C, the heat required is 625 calories (Q1) and 20,000 calories for melting (Q2), totaling 20,625 calories.
- 😀 A second example involves 200 grams of ice at -5°C being heated to 100°C. The total heat required for this process is 36,500 calories, calculated in three stages: heating the ice (Q1), melting the ice (Q2), and heating the resulting water to 100°C (Q3).
- 😀 The importance of using appropriate formulas for different stages of heat transfer is emphasized to ensure accurate calculations of energy needed for phase changes and temperature changes.
Q & A
What is the main topic of the video script?
-The main topic of the video is about understanding the changes in the states of matter (solid, liquid, gas) when heat is applied, and how to calculate the heat required for these changes.
What formula is used to calculate heat in the video?
-The formula used to calculate heat is Q = m * c * ΔT, where Q is the heat, m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
What is the significance of the graph shown in the video?
-The graph illustrates the phase change of water from solid (ice) to liquid (water), and then to gas (steam), showing the temperature changes during these processes.
How is the heat calculation for the phase change of ice to water explained?
-For the phase change of ice to water, two processes are involved: heating the ice (Q1) and the melting of the ice (Q2). Q1 uses the specific heat of ice, and Q2 uses the latent heat of fusion.
What is the formula used for calculating the heat required for melting ice?
-The formula used for melting ice is Q = m * L, where m is the mass of the ice and L is the latent heat of fusion.
How is heat calculated for the process of water turning into steam?
-For water turning into steam, the formula Q = m * L is used, where L is the latent heat of vaporization, and the temperature remains constant at 100°C during this process.
What is the total heat required to change ice at -5°C to water at 0°C, as explained in the example?
-In the example, the total heat required is 20,605 calories. This is the sum of Q1 (625 calories) for heating the ice and Q2 (20,000 calories) for melting the ice.
What changes occur in the second example when ice is heated to water at 100°C?
-In the second example, the ice undergoes three processes: heating from -5°C to 0°C (Q1), melting from ice to water (Q2), and heating the water from 0°C to 100°C (Q3). The total heat required is 36,500 calories.
What is the importance of using different formulas for different phases of matter?
-Different formulas are used because the specific heat capacity and latent heat values differ depending on the phase of the substance. The heating of a solid, the melting of a solid, and the vaporization of a liquid all require different calculations.
What is the role of temperature in the heat calculation process described in the video?
-Temperature plays a critical role in determining the amount of heat required for a substance to undergo phase changes or temperature changes. The change in temperature (ΔT) is used in the formula Q = m * c * ΔT for temperature changes, and the temperature remains constant during phase changes like melting or vaporization.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

Physics 22 Introduction to Heat & Temperature (6 of 6) Change of Phase & Latent Heat
Heating Curve and Cooling Curve of Water - Enthalpy of Fusion & Vaporization

TROCAS DE CALOR COM MUDANÇA DE FASE - TERMOLOGIA - Aula 8 - Prof. Boaro

Calorimetria - Aula 03 (Calor Latente)

Calorimetría: 2 problemas (sin cambio de estado) | Biofísica CBC | Física En Segundos (por Aníbal)

Phase Changes: Exothermic or Endothermic?
5.0 / 5 (0 votes)
