Izomerie optică – chiralitatea | Lectii-Virtuale.ro
Summary
TLDRThe transcript explores the concept of chemical isomerism, explaining various types such as constitutional, chain, position, and functional isomers. It delves into stereoisomerism, including conformational and configurational isomers, discussing how these molecules differ in structure and properties despite having the same molecular formula. It also introduces chirality in molecules, highlighting how certain molecules cannot be superimposed on their mirror images, leading to the formation of enantiomers. The discussion extends to the importance of asymmetric carbon atoms in creating chiral molecules and the practical implications of chirality in daily life, such as in objects like gloves and shoes.
Takeaways
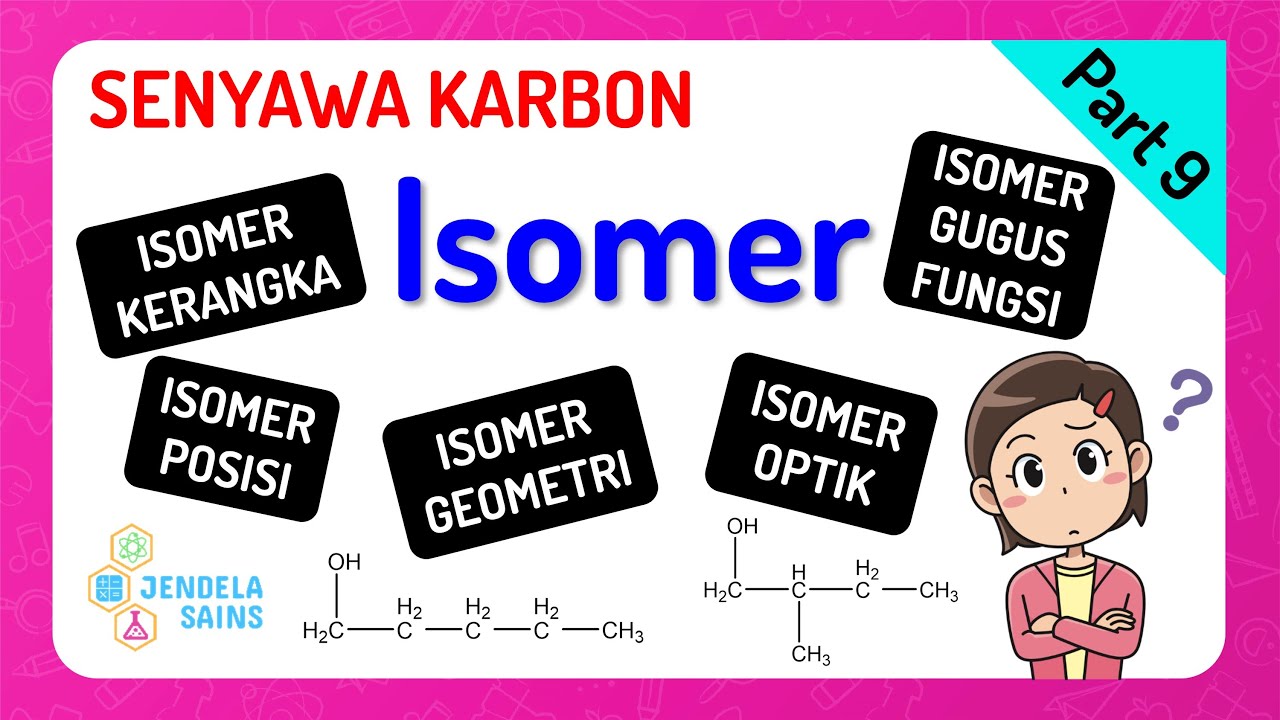
- 😀 Isomers are chemical compounds with the same molecular formula but different properties.
- 😀 There are two main categories of isomers: constitutional isomers and stereoisomers.
- 😀 Constitutional isomers differ in the connectivity or arrangement of atoms in the molecule.
- 😀 Stereoisomers differ in the spatial arrangement of atoms but share the same connectivity.
- 😀 Structural isomers can be further divided into chain isomers, position isomers, and functional isomers.
- 😀 Chain isomers differ in the arrangement of carbon atoms in the molecule.
- 😀 Position isomers differ in the position of functional groups within the molecule.
- 😀 Functional isomers have the same molecular formula but differ in the types of functional groups they contain.
- 😀 Stereoisomers can be further divided into conformational isomers and configurational isomers.
- 😀 Chiral objects, such as human hands, are non-superimposable on their mirror images, and this concept applies to molecules as well.
- 😀 A chiral molecule often contains an asymmetric carbon atom bonded to four different groups, and this carbon is known as a chiral center or stereocenter.
Q & A
What is the meaning of isomerism in chemistry?
-Isomerism refers to the occurrence of compounds with the same molecular formula but different structural arrangements, resulting in distinct chemical properties.
What are constitutional isomers?
-Constitutional isomers are compounds that have the same molecular formula but differ in the connectivity of their atoms, leading to different structural arrangements.
What is the difference between chain isomers and position isomers?
-Chain isomers differ in the arrangement of carbon atoms in the main chain, while position isomers differ in the position of a functional group on the carbon chain.
Can you give an example of functional group isomers?
-An example of functional group isomers includes ethanol and dimethyl ether. They have the same molecular formula but different functional groups and, therefore, belong to different chemical classes.
What are stereoisomers?
-Stereoisomers are compounds that have the same molecular and structural formulas but differ in the spatial arrangement of atoms. These can be further categorized into conformational isomers and configurational isomers.
What are the two main categories of stereoisomers?
-Stereoisomers are divided into conformational isomers, which can convert into one another through rotation around single bonds, and configurational isomers, which cannot convert without breaking and reforming bonds.
What is chirality in molecules?
-Chirality refers to a property of molecules that are non-superimposable on their mirror image, much like left and right hands. A molecule with chirality typically has a carbon atom bonded to four different atoms or groups.
What is the role of asymmetric carbon in chirality?
-An asymmetric carbon, or chiral center, is a carbon atom bonded to four different atoms or groups, making the molecule chiral. This gives rise to mirror-image stereoisomers called enantiomers.
What is the difference between enantiomers and diastereoisomers?
-Enantiomers are stereoisomers that are mirror images of each other but cannot be superimposed. Diastereoisomers, on the other hand, are stereoisomers that are not mirror images and differ in the spatial arrangement of atoms.
How are chirality and the concept of left and right hands related?
-Chirality is similar to the concept of left and right hands because, just like the hands are mirror images but cannot be superimposed, chiral molecules have mirror images that are non-superimposable, known as enantiomers.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)