Physics - E&M: Ch 35.1 Coulumb's Law Explained (1 of 28) Why do Charged Objects Repel or Attract?
Summary
TLDRIn this video, the concept of Coulomb's law is explored, focusing on the interactions between charged objects. The video explains how positive and negative charges interact, with like charges repelling and opposite charges attracting. It dives into the atomic structure, with protons, neutrons, and electrons, and the role of electrons in charge transfer. The influence of electric fields around charged objects is discussed, illustrating how these fields affect other charges within their sphere of influence. The video sets the stage for a deeper understanding of Coulomb's law and its mathematical formulation.
Takeaways
- 😀 Coulomb's law explains how charged objects interact, either attracting or repelling each other.
- 😀 Objects with the same type of charge (either both positive or both negative) will repel each other.
- 😀 Opposite charges (positive and negative) will attract each other.
- 😀 All objects are made of atoms, which consist of protons (positive charge), neutrons (neutral), and electrons (negative charge).
- 😀 Electrons can move between objects, but protons are fixed in the atomic nucleus and cannot move.
- 😀 If an object has more electrons than protons, it becomes negatively charged.
- 😀 If an object has more protons than electrons, it becomes positively charged.
- 😀 In conductors, excess charge typically resides on the surface of the object, while insulators may distribute charge throughout.
- 😀 A charged object creates an electric field around it, affecting the space nearby.
- 😀 For positive charges, the electric field radiates outward, repelling nearby positive charges and attracting negative ones.
- 😀 For negative charges, the electric field points inward, attracting nearby positive charges and repelling negative ones.
Q & A
What is Coulomb's Law?
-Coulomb's Law describes how charged objects interact with each other, explaining that like charges repel and opposite charges attract. It quantifies the force between two charged objects based on their charges and the distance between them.
What types of particles make up atoms?
-Atoms are made of protons, neutrons, and electrons. Protons are positively charged, neutrons are neutral, and electrons are negatively charged.
Why do objects with the same charge repel each other?
-Objects with the same charge repel each other because their electric fields interact in such a way that they exert a force pushing them apart.
What happens when two objects with opposite charges interact?
-Objects with opposite charges attract each other because their electric fields pull them toward one another, leading to an attractive force.
Why can't protons be transferred between objects?
-Protons are locked in the nucleus of atoms and cannot be moved. Only electrons, which are outside the nucleus, can be transferred between objects.
How do electrons influence the charge of an object?
-The charge of an object depends on the balance between protons and electrons. If there are more electrons than protons, the object becomes negatively charged, and if there are more protons than electrons, the object becomes positively charged.
What is the difference between conductors and insulators in terms of charge distribution?
-In conductors, excess charges tend to reside near the surface, while in insulators, the charge can be spread throughout the entire object.
What is an electric field, and how does it relate to charged objects?
-An electric field is the space around a charged object that influences other charged objects within it. The electric field lines point away from positive charges and toward negative charges, causing other charges to either be repelled or attracted.
How do positive and negative charges behave within an electric field?
-Positive charges are repelled by other positive charges and attracted to negative charges, while negative charges are attracted to positive charges and repelled by other negative charges.
What is the mathematical form of Coulomb's Law?
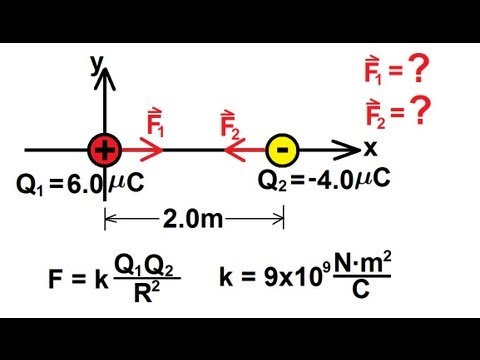
-The mathematical form of Coulomb's Law quantifies the force between two charges, factoring in the magnitudes of the charges and the distance between them. It is expressed as F = k * (q1 * q2) / r^2, where F is the force, k is Coulomb's constant, q1 and q2 are the charges, and r is the distance between them.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

Fisika kelas 12 | Listrik Statis (part 1) Muatan listrik dan gaya coulomb

IPA Kelas 9 : Hukum Coulomb dan Cara Menyelesaikan Soal-Soalnya

Tema 01 - A Carga Elétrica e o Spin | Experimentos - Lei de Coulomb

01. Conceptos Básicos de la Electrostática (Introducción)

Hukum Coulomb Kelas 12
Physics 35 Coulomb's Law (1 of 8)
5.0 / 5 (0 votes)
