14 CYCLIC PROCESS | THERMODYNAMICS | IIT ADVANCED | JEE MAIN | CHEMISTRY CLASS 11 | OLYMPIAD
Summary
TLDRThis video explains the concept of cyclic processes in thermodynamics, focusing on the first law of thermodynamics and the relationship between pressure, volume, and work. It covers both clockwise and counterclockwise cycles, showing how work is calculated based on the area under the P-V graph. Key concepts include energy conservation, heat exchange, and the effects of different process types (like isothermal). The video also highlights the importance of understanding reversible and irreversible processes, providing insights on calculating work done and exploring the thermodynamic cycles in depth.
Takeaways
- 😀 The First Law of Thermodynamics was discussed, focusing on energy conservation in thermodynamic processes.
- 😀 Cyclic processes were introduced, explaining that they are combinations of various thermodynamic processes.
- 😀 The concept of 'work done' in cyclic processes was explained using clockwise and counter-clockwise loops, where work done is represented by the area enclosed by the cycle on a pressure-volume graph.
- 😀 In cyclic processes, the change in internal energy (ΔU) can be zero, but work and heat may still be exchanged.
- 😀 Clockwise cycles represent compression processes where work done is negative, while counter-clockwise cycles correspond to expansion processes with positive work done.
- 😀 The speaker emphasized that the total work done in a cyclic process is the net area enclosed by the cycle on a pressure-volume graph.
- 😀 The relationship between pressure, volume, and temperature during cyclic processes was highlighted, demonstrating how to calculate work and other parameters.
- 😀 Various thermodynamic processes like isothermal processes and their detailed characteristics were briefly covered, with a focus on the work calculation and energy changes in each type.
- 😀 The concept of heat energy transfer in cyclic processes was explained, showing how heat is both absorbed and released during expansion and compression phases.
- 😀 The importance of calculating work in thermodynamic cycles was stressed, especially in the context of objective-type questions, with examples provided on how to calculate work done using given pressure-volume data.
Q & A
What is a cyclic process in thermodynamics?
-A cyclic process in thermodynamics is a sequence of thermodynamic changes where a system returns to its initial state. In this process, the system undergoes a series of transformations, and the net work done can be calculated based on the area enclosed by the cycle on a pressure-volume graph.
How does the first law of thermodynamics apply to cyclic processes?
-In a cyclic process, the first law of thermodynamics states that the change in internal energy (ΔU) is zero. This implies that the work done by the system equals the heat absorbed or released, as there is no change in the internal energy during the cycle.
How is work calculated in cyclic processes?
-The work done in cyclic processes is determined by calculating the area enclosed by the cycle on the pressure-volume (P-V) graph. If the cycle is clockwise, the work is positive, and if it is counterclockwise, the work is negative.
What does it mean when a cyclic process is clockwise versus counterclockwise?
-In a clockwise cyclic process, the system performs work (expansion), which results in positive work. In a counterclockwise cyclic process, the system absorbs work (compression), which results in negative work.
Why is the area under the P-V curve important in cyclic processes?
-The area under the pressure-volume curve in a P-V graph represents the work done during the cyclic process. Positive work corresponds to the area under the curve for expansion, while negative work corresponds to the area for compression.
What is the role of heat in cyclic processes according to the first law of thermodynamics?
-According to the first law of thermodynamics, the heat added to a system during a cyclic process equals the work done by the system, as the change in internal energy is zero. This is an important concept in heat engines, where heat conversion into work is analyzed.
What happens to the internal energy in a cyclic process?
-In a cyclic process, the internal energy of the system remains unchanged. This is because the system returns to its initial state after completing the cycle, making the net change in internal energy zero.
What is meant by the term 'reversible process' in thermodynamics?
-A reversible process in thermodynamics is one that can be reversed by an infinitesimally small change in conditions, returning the system and surroundings to their original states. In cyclic processes, reversibility ensures no net energy loss during the cycle.
How do isothermal processes fit into the discussion of cyclic processes?
-Isothermal processes, where temperature remains constant, are one type of process that can be part of a cyclic process. In such processes, the work done is entirely dependent on the heat absorbed or released, as the temperature does not change.
Why is understanding the work done in cyclic processes important for real-world applications like heat engines?
-Understanding the work done in cyclic processes is essential for optimizing the efficiency of heat engines, which rely on converting heat energy into mechanical work. By calculating the work done based on P-V graphs, engineers can design more efficient engines and refrigeration systems.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频
Hukum 1 Termodinamika : Termodinamika Fisika Kelas 11 - Part 1
12th Physics | Chapter 4 | Thermodynamics | Lecture 2 | Maharashtra Board |

1ª LEI DA TERMODINÂMICA | Resumo de Física para o Enem

Thermodynamics Class 11 in 5 Minutes | Chemistry | Quick Revision | NEET, JEE & CBSE |
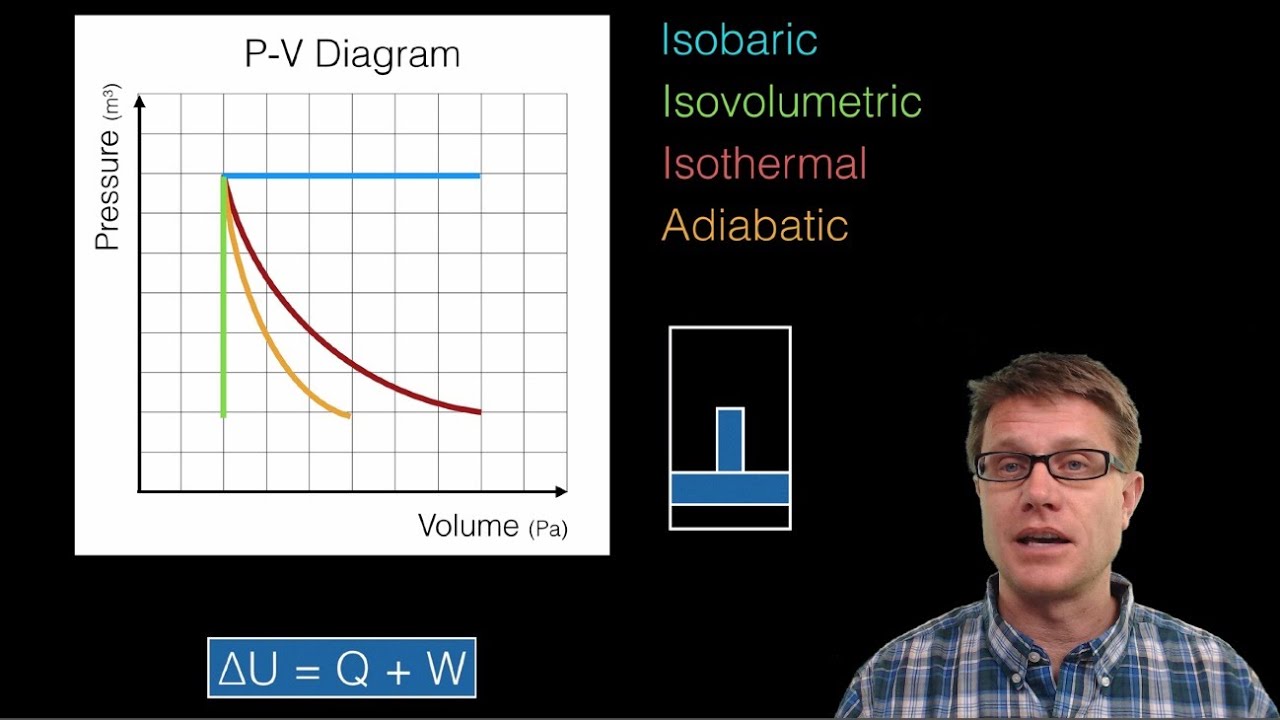
Thermodynamics and P-V Diagrams

Materi Fisika Kelas 11 Termodinamika
5.0 / 5 (0 votes)
