Solutions, Percent by Mass and Volume
Summary
TLDRThis chemistry lecture explores solutions, focusing on how to calculate concentration using percent by mass and percent by volume. It explains key concepts such as solvent, solute, and how solution concentration can vary from dilute to concentrated. The lecture includes formulas for percent by volume and mass, demonstrating how to solve example problems, such as determining the ethanol content in a solution or calculating the mass of glucose required for a specific concentration. Overall, the video provides a clear, practical understanding of these essential concepts in solution chemistry.
Takeaways
- 😀 Solutions are homogeneous mixtures of two or more substances, with water often acting as the solvent.
- 😀 The solvent is usually in greater quantity than the solute, although this isn't always the case.
- 😀 A solution can be described as dilute (small amount of solute) or concentrated (large amount of solute).
- 😀 Percent by volume represents the volume of solute in 100 mL of solution, commonly used for liquid solutions.
- 😀 An example of percent by volume: a 70% rubbing alcohol solution means 70 mL of the solution is isopropyl alcohol.
- 😀 The formula for percent by volume is: (Volume of Solute / Volume of Solution) * 100.
- 😀 An example calculation: 80 mL of ethanol diluted to 250 mL gives a percent by volume of 32%.
- 😀 Percent by mass measures the mass of solute in relation to the total mass of the solution.
- 😀 The formula for percent by mass is: (Mass of Solute / Total Mass of Solution) * 100.
- 😀 An example of percent by mass: If 7 g of NaCl is dissolved in 80 g of solution, the percent by mass is 8.75%.
- 😀 To make a 2000 g glucose solution at 2.8% by mass, 56 g of glucose is required, with water added to reach the total mass.
Q & A
What is a solution?
-A solution is a homogeneous mixture made up of two or more substances. The solvent is typically the substance in the larger quantity, and the solute is the substance in the lesser quantity.
What is the difference between a dilute solution and a concentrated solution?
-A dilute solution contains a small amount of solute relative to the solvent, while a concentrated solution contains a large amount of solute compared to the solvent.
How is the concentration of a solution expressed when both components are liquids?
-The concentration is expressed as percent by volume, which tells you the volume of solute in 100 mL of the total solution.
How do you calculate percent by volume?
-Percent by volume is calculated using the formula: (Volume of Solute / Volume of Solution) * 100.
What is an example of calculating percent by volume?
-If 80 mL of ethanol is diluted to 250 mL with water, the percent by volume is calculated as: (80 / 250) * 100 = 32%. This means that in 100 mL of the solution, 32 mL is ethanol.
How is percent by mass different from percent by volume?
-Percent by mass is used when a solid solute is dissolved in a liquid solvent, and it is calculated using the mass of solute and the total mass of the solution, while percent by volume is used for liquid-liquid solutions.
How do you calculate percent by mass?
-Percent by mass is calculated using the formula: (Mass of Solute / Total Mass of Solution) * 100.
What is an example of calculating percent by mass?
-If 7 g of NaCl is dissolved in 80 g of solution, the percent by mass is calculated as: (7 / 80) * 100 = 8.75%. This means that in 100 g of the solution, 8.75 g is NaCl.
How do you solve for the mass of solute if given a desired mass of solution and percent by mass?
-To solve for the mass of solute, you can rearrange the percent by mass formula to: Mass of Solute = (Percent by Mass * Total Mass of Solution) / 100.
How much glucose is needed to prepare 2,000 g of a 2.8% glucose solution?
-To prepare 2,000 g of a 2.8% glucose solution, you need 56 g of glucose. This is calculated using the formula: (2.8 * 2,000) / 100 = 56 g.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

Concentração das Soluções em Porcentagem

Konsentrasi Larutan bag 1
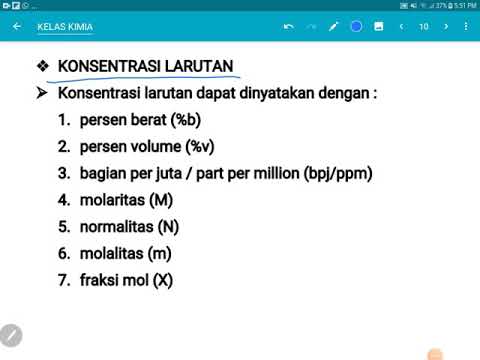
Kelas Kimia : Konsentrasi Larutan (% berat, % volume, ppm / bpj)

Stoikiometri (4) | Menentukan Kadar Unsur dalam Senyawa | Kimia Kelas 11

How to Find the Percent Composition by Mass for a Compound

Percent Compositions of Compound| Grade 9 Science Quarter 2 Week 8 | DepEd MELC-based
5.0 / 5 (0 votes)
