How to Find the Percent Composition by Mass for a Compound
Summary
TLDRIn this video, viewers are taught how to calculate the percent composition by mass of elements in compounds. Using examples like CO2, KNO3, and Ca(OH)2, the process involves finding the molar mass of each element, multiplying by its number of atoms, and dividing by the total molar mass of the compound. The tutorial explains how to express these values as percentages and emphasizes the significance of percent composition in determining empirical and molecular formulas. This concept is critical in chemistry for analyzing the ratios of elements in various substances.
Takeaways
- 😀 Percent composition by mass refers to the percentage of each element in a compound based on its molar mass.
- 😀 The formula for percent composition is: (Mass of element / Molar mass of compound) × 100.
- 😀 The example of carbon dioxide (CO2) shows that the percent of carbon is 27.29% and the percent of oxygen is 72.71%.
- 😀 To calculate the percent composition, first find the molar mass of the element and compound using the periodic table.
- 😀 The percent composition of each element must add up to 100% (with minor rounding errors).
- 😀 In potassium nitrate (KNO3), the percent composition for potassium is 38.67%, nitrogen is 13.86%, and oxygen is 47.47%.
- 😀 When calculating the percent composition for compounds like Ca(OH)2, you must consider the subscripts for each element and multiply accordingly.
- 😀 For calcium hydroxide, the molar mass of the compound is 74.09 grams per mole, and the percent of hydrogen is 2.73%.
- 😀 The video emphasizes practicing percent composition calculations for different compounds to fully grasp the concept.
- 😀 The purpose of calculating percent composition is to determine the ratio of elements in a compound, which is useful for finding empirical and molecular formulas.
Q & A
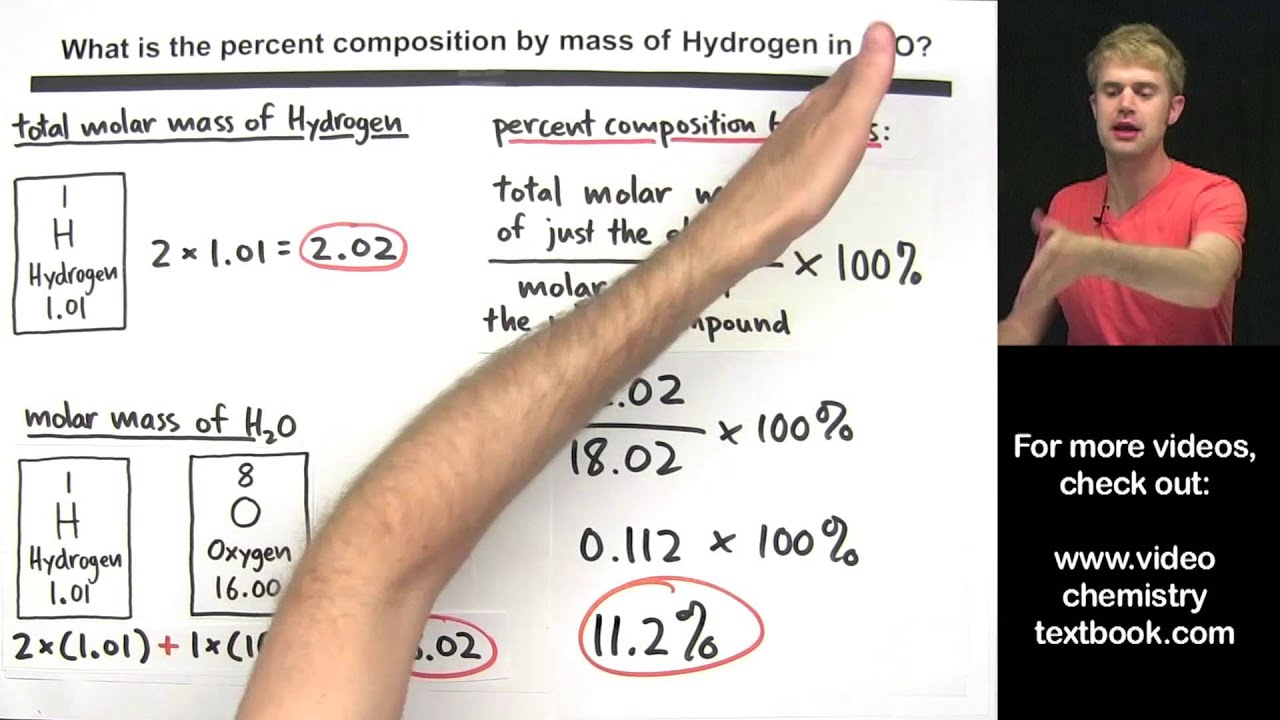
What is percent composition by mass?
-Percent composition by mass is the percentage of the total mass of a compound that is contributed by each element in that compound. It is calculated by dividing the mass of the individual element by the molar mass of the compound, then multiplying by 100.
How do you calculate the percent composition of carbon in CO2?
-To calculate the percent composition of carbon in CO2, you take the molar mass of carbon (12.01 g/mol), multiply it by the number of carbon atoms (which is 1 in CO2), and divide by the molar mass of CO2 (44.01 g/mol). Then, multiply the result by 100 to get the percentage, which is 27.29%.
What does the formula for percent composition look like?
-The formula for percent composition is: Percent Composition = (Mass of Element / Molar Mass of Compound) * 100.
Why do the percentages for carbon and oxygen in CO2 add up to almost 100%?
-The percentages for carbon and oxygen in CO2 add up to almost 100% because they represent the total mass of the molecule, and there are no other elements in the compound. Minor rounding errors may cause the total to be slightly less than 100%.
How do you calculate the percent composition for oxygen in CO2?
-For oxygen in CO2, you take the molar mass of oxygen (16.00 g/mol), multiply it by 2 (since there are two oxygen atoms), and divide by the molar mass of CO2 (44.01 g/mol). Then, multiply by 100 to get the percentage, which is 72.71%.
What is the purpose of calculating percent composition in chemistry?
-Calculating percent composition helps determine the ratio of different elements in a compound. This information is useful for determining empirical and molecular formulas, as well as for conducting laboratory experiments and reactions.
How do you find the percent composition of potassium in KNO3?
-To find the percent composition of potassium in KNO3, you use the molar mass of potassium (39.10 g/mol), multiply it by 1 (since there is one potassium atom), and divide by the total molar mass of KNO3 (101.11 g/mol). Multiply by 100 to get the percentage, which is 38.67%.
How is the percent composition of nitrogen calculated in KNO3?
-To calculate the percent composition of nitrogen in KNO3, you take the molar mass of nitrogen (14.01 g/mol), divide it by the total molar mass of KNO3 (101.11 g/mol), and multiply by 100. This gives a percentage of 13.86%.
What is the percent composition of oxygen in KNO3?
-The percent composition of oxygen in KNO3 is calculated by taking the molar mass of oxygen (16.00 g/mol), multiplying it by 3 (because there are three oxygen atoms), dividing by the molar mass of KNO3 (101.11 g/mol), and multiplying by 100. The result is 47.47%.
How is the percent composition of hydrogen calculated in Ca(OH)2?
-To calculate the percent composition of hydrogen in Ca(OH)2, you first calculate the molar mass of the compound, which is 74.09 g/mol. The mass of hydrogen is found by multiplying the molar mass of hydrogen (1.01 g/mol) by 2 (since there are two hydrogen atoms). The percent composition of hydrogen is then calculated by dividing the mass of hydrogen by the molar mass of Ca(OH)2 and multiplying by 100, resulting in 2.73%.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Stoikiometri (4) | Menentukan Kadar Unsur dalam Senyawa | Kimia Kelas 11

Percent Compositions of Compound| Grade 9 Science Quarter 2 Week 8 | DepEd MELC-based
Percent Composition By Mass

Empirical Formula from Percent Composition

S9Q2W8 | PERCENT COMPOSITION OF COMPOUNDS
How to Find the Percent Composition by Mass for Zn(OH)2 (Zinc hydroxide)
5.0 / 5 (0 votes)