Stoikiometri
Summary
TLDRThis video provides a concise introduction to stoichiometry, the science of calculating substance quantities in chemical reactions. It explains the core concepts of moles, including how to convert between moles, mass, volume, and particle number using fundamental principles and formulas. A practical example illustrates how to prepare a potassium permanganate solution, detailing the steps to calculate required mass based on molarity and volume. This foundational knowledge sets the stage for understanding more advanced stoichiometric laws, encouraging viewers to continue their learning journey.
Takeaways
- 😀 Stoichiometry is the science used to calculate the quantities of substances involved in chemical reactions.
- 😀 Key units in stoichiometry include weight, volume, concentration, and particle count.
- 😀 Calculations in stoichiometry are based on the concept of moles.
- 😀 To find moles from weight, divide the weight by the relative atomic mass (Mr).
- 😀 The volume of one mole of gas at STP is approximately 22 liters.
- 😀 To convert from moles to particles, use Avogadro's number (6.02 x 10^23).
- 😀 To calculate weight from moles, multiply the number of moles by the relative atomic mass.
- 😀 For solutions, molarity (M) can be found by dividing moles by volume in liters.
- 😀 Example: To prepare a 1M solution of potassium permanganate, calculate the moles required based on the volume.
- 😀 The total mass needed can be calculated using the relative atomic masses of the components in the compound.
Q & A
What is stoichiometry?
-Stoichiometry is the science used to calculate the quantities of substances involved in chemical reactions, using units such as weight, volume, and particle count.
What is the significance of the mole concept in stoichiometry?
-The mole concept is fundamental in stoichiometry, as it allows for the conversion between mass, volume, and the number of particles based on the relationships defined by chemical reactions.
How do you calculate moles from weight?
-To calculate moles from weight, divide the weight of the substance by its molar mass (relative atomic mass).
What is Avogadro's number and how is it used?
-Avogadro's number (approximately 6.02 x 10^23) is used to determine the number of particles in one mole of a substance.
What is the formula to find the volume of a gas at STP?
-The volume of a gas at standard temperature and pressure (STP) can be found by multiplying the number of moles by 22 liters per mole.
How do you calculate molarity?
-Molarity is calculated by dividing the number of moles of solute by the volume of the solution in liters.
In the example provided, how many grams of KMnO4 are needed to prepare a 1M solution in 500 mL?
-To prepare a 1M solution of KMnO4 in 500 mL, you need to weigh 79 grams of KMnO4.
What is the molecular weight of potassium permanganate (KMnO4)?
-The molecular weight of potassium permanganate (KMnO4) is calculated as 158 grams per mole.
What is the process for determining the mass of a substance needed for a specific molarity?
-To determine the mass of a substance needed for a specific molarity, calculate the moles required using the formula (molarity x volume in liters) and then multiply by the substance's molar mass.
What will be discussed in the next video?
-The next video will cover the basic laws of stoichiometry.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

ATURAN DALAM PERHITUNGAN PERUBAHAN ENTALPI
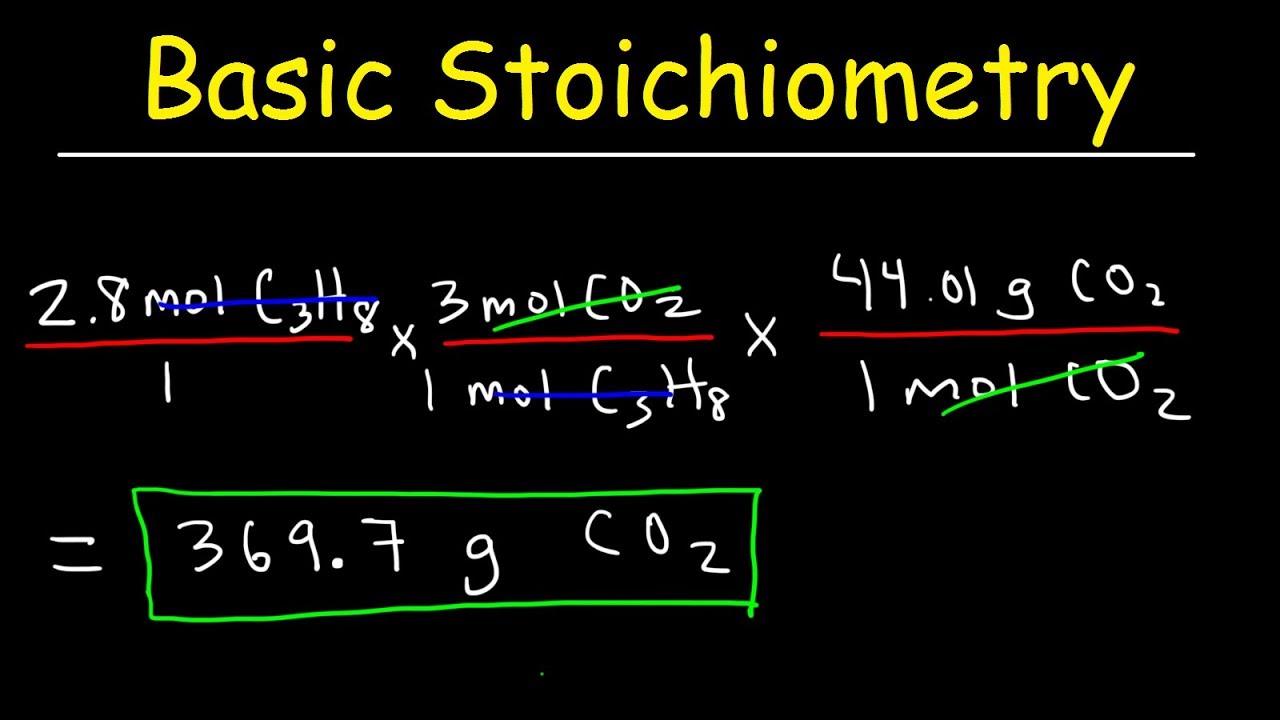
Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems

STOIKIOMETRI (Konsep Mol)
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6

Stoichiometry Made Easy: Stoichiometry Tutorial Part 1

BAB 5 REAKSI - REAKSI KIMIA DAN DINAMIKANYA PART 1 (IPA Kelas 9 Kurikulum Merdeka)
5.0 / 5 (0 votes)
