GCSE Chemistry - Balancing Chemical Equations #4
Summary
TLDRThis video explains the fundamentals of balancing chemical equations, highlighting the difference between word equations and symbol equations. It illustrates how reactants, such as methane and oxygen, transform into products like carbon dioxide and water. The video emphasizes the importance of having the same number of each type of atom on both sides of the equation. Through examples, it demonstrates the process of trial and error in balancing equations, adhering to rules that prohibit changing subscripts and requiring whole numbers. This engaging tutorial is essential for understanding chemical reactions and stoichiometry.
Takeaways
- 😀 Chemical equations are essential for understanding chemical reactions.
- 🔍 A word equation describes reactants and products using their names.
- ⚗️ The symbol equation uses chemical symbols to represent reactants and products.
- 🌬️ Oxygen exists as O2, meaning it consists of two atoms.
- ⚖️ Balancing chemical equations requires equal numbers of each atom on both sides.
- 🔄 Changing small numbers in chemical formulas alters the substance, which is not allowed.
- 1️⃣ The large numbers in front of compounds represent how many molecules are present.
- 🧪 Balancing involves trial and error to achieve the correct atom counts.
- 🔢 Whole numbers must be used when balancing equations; fractional molecules are not permitted.
- 🧮 It's helpful to balance the least common elements first for efficiency.
Q & A
What are the reactants in the chemical reaction described in the video?
-The reactants in the chemical reaction are methane (CH4) and oxygen (O2).
What are the products formed when methane burns in oxygen?
-The products formed are carbon dioxide (CO2) and water (H2O).
What does the arrow in a chemical equation represent?
-The arrow represents that the reactants react completely to form the products.
Why is it important to balance chemical equations?
-Balancing chemical equations is important because it ensures that the total number of each type of atom is the same on both sides of the equation, adhering to the law of conservation of mass.
How do you represent oxygen in a chemical equation?
-Oxygen is represented as O2 in a chemical equation because it exists as a diatomic molecule made up of two atoms.
What are the rules for balancing chemical equations?
-The rules include not changing the small numbers (subscripts) of the chemical formulas, only changing the big numbers (coefficients) in front of the compounds, and maintaining whole numbers.
In the example provided, how is the equation balanced?
-In the methane combustion example, a coefficient of 2 is placed in front of O2, and the number of water molecules is increased from 1 to 2 to balance the hydrogen and oxygen atoms.
What elements were balanced first in the second example involving sulfuric acid?
-In the second example, sulfur and sodium were balanced first, as sulfur was already balanced with one atom on either side.
How did the presenter suggest fixing the imbalance of sodium in the sulfuric acid reaction?
-To fix the imbalance of sodium, the presenter suggested adding a coefficient of 2 in front of sodium hydroxide to obtain two sodium atoms on the left side.
What final check should be done after balancing a chemical equation?
-After balancing a chemical equation, it's important to double-check to ensure that the number of each type of atom is the same on both sides of the equation.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

Introduction to Balancing Chemical Equations
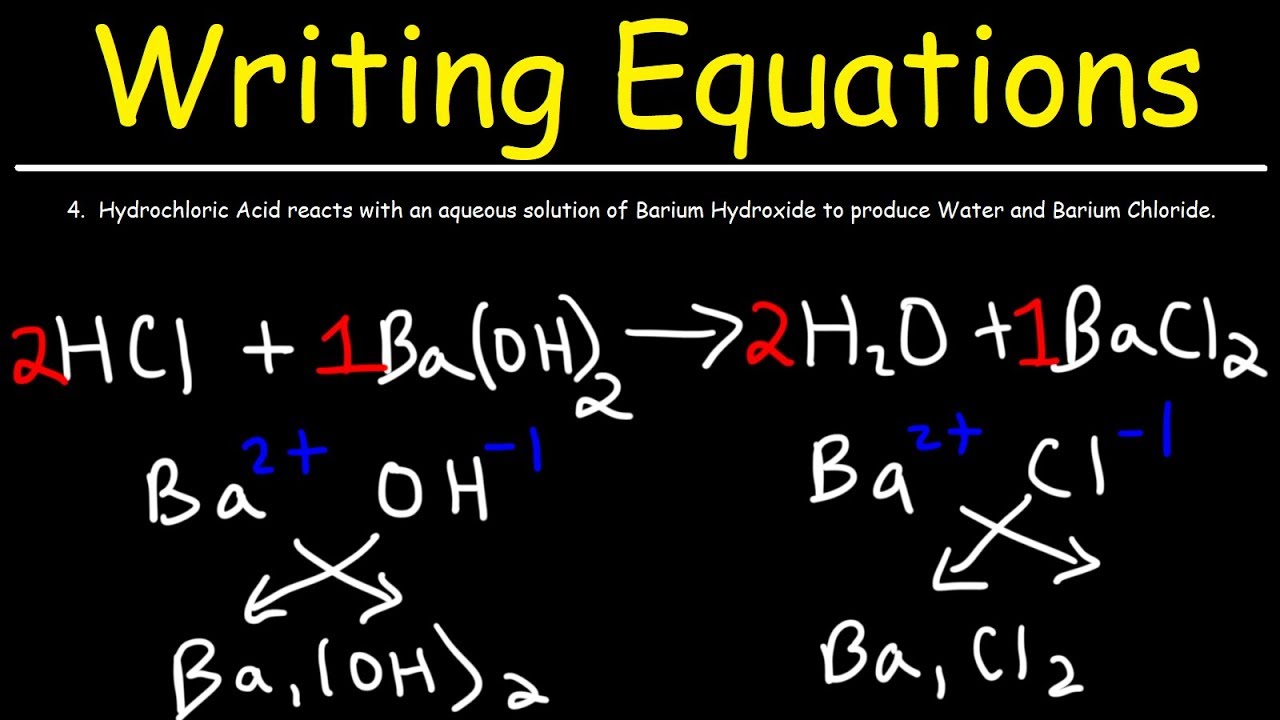
How To Write Chemical Equations From Word Descriptions

PERSAMAAN REAKSI DAN CARA PENYETARAANNYA ( KIMIA SMA KELAS 10 )
How to Write Chemical Equations

CBSE Class 10 Science - 1 | Chemical Reactions and Equations | Full Chapter | NCERT Animation

Persamaan reaksi dan penyetaraan reaksi kimia - Kimia SMA kelas 10 semester 2
5.0 / 5 (0 votes)
