Grade 10 SCIENCE | Gay-Lussac's Law
Summary
TLDRThe video script discusses Gay-Lussac's Law, which describes the direct proportionality between pressure and temperature for a given amount of gas at constant volume. The presenter explains the law's formula, ( P1/T1 = P2/T2 ), where P stands for pressure and T for temperature in Kelvin. The importance of using consistent units and converting Celsius to Kelvin for temperature measurements is highlighted. Two problems are solved using the law: one involving a gas cylinder with varying pressures and temperatures, and another concerning the pressure change in a helium-filled balloon with an increase in temperature. The video aims to help viewers understand the relationship between pressure and temperature, emphasizing the need for step-by-step problem-solving and accurate unit conversion.
Takeaways
- 📚 Gay-Lussac's Law is not part of the original video series but was requested by viewers, showing the relationship between pressure and temperature.
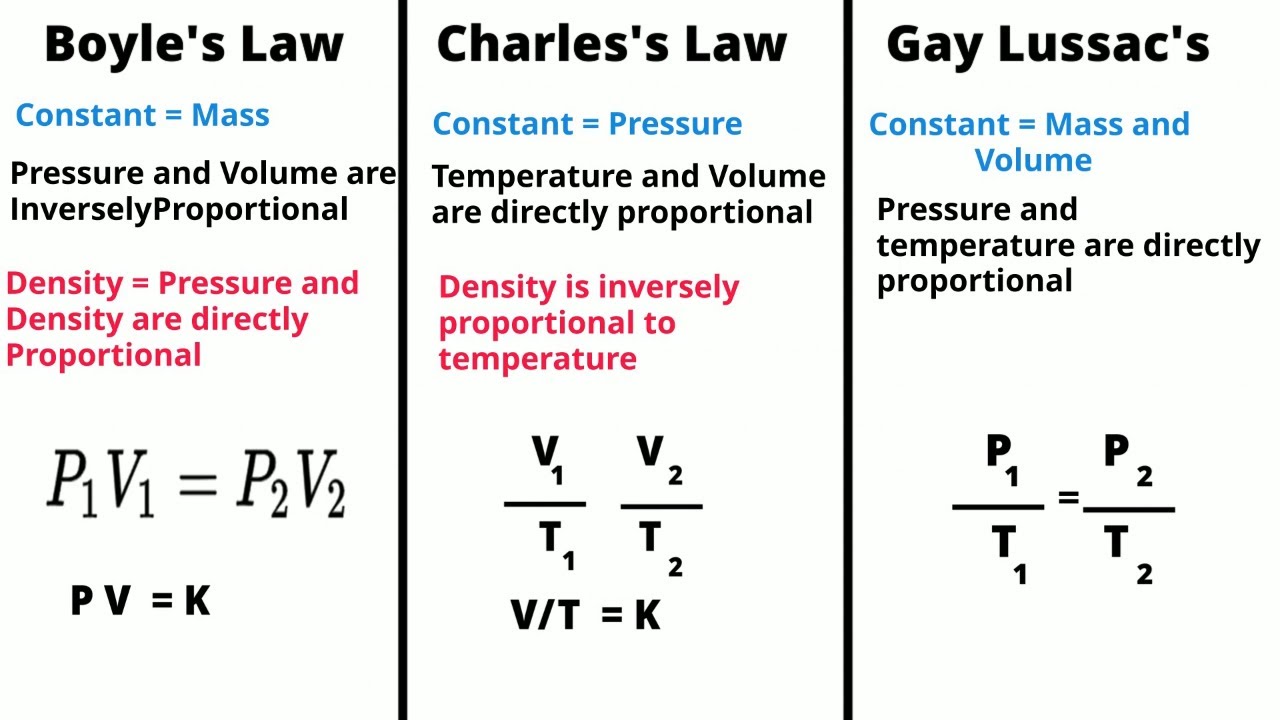
- 🔗 The law is similar to Charles' Law and is often discussed alongside Boyle's Law, with a link provided for further review.
- 📐 The general form of Gay-Lussac's Law is p1/t1 = p2/t2, where p represents pressure and t represents temperature.
- 📉 Subscripts 1 and 2 refer to the initial and final states of a system, which are crucial for problem-solving.
- 🔍 Units for pressure can vary (e.g., atmospheres, mmHg, kPa), but temperature must be in Kelvin, converting from Celsius if necessary.
- ⚖️ Direct proportionality means that if temperature increases, pressure increases, and vice versa, assuming volume and the amount of substance are constant.
- ✅ The importance of understanding the relationships between pressure and temperature is emphasized for accurate problem-solving.
- 🔢 Problem-solving involves converting temperatures to Kelvin when given in Celsius, and using the correct formula for Gay-Lussac's Law.
- 🌡️ Example problem: A gas cylinder with an initial pressure and temperature is used to find the new temperature at a different pressure, requiring unit conversion and equation manipulation.
- 🎈 Another example involves a mylar balloon with helium gas, where the pressure change is calculated given a temperature increase from 22°C to 45°C.
- 📈 The expected outcome of the second problem is a pressure greater than the initial 107 kilopascals due to the direct relationship between temperature and pressure.
- 📝 The final pressure for the helium balloon is calculated to be 115 kilopascals, demonstrating the application of Gay-Lussac's Law in real-world scenarios.
Q & A
What is Gay-Lussac's Law?
-Gay-Lussac's Law relates the pressure and temperature of a gas when the volume and the amount of gas are held constant. It states that the pressure of a gas is directly proportional to its temperature in Kelvin, given by the formula p1/T1 = p2/T2, where p is pressure and T is temperature.
How is Gay-Lussac's Law similar to Charles's Law?
-Both Gay-Lussac's Law and Charles's Law describe the relationship between pressure and temperature in a gas. However, Charles's Law specifically deals with the volume and temperature relationship at constant pressure, while Gay-Lussac's Law focuses on the pressure and temperature relationship at constant volume.
What are the units typically used for pressure in problems related to Gay-Lussac's Law?
-Pressure can be expressed in various units such as atmospheres, millibars, torr (millimeter mercury), or kilopascals. The specific unit used is not critical as long as the problem and the answer use the same unit.
How do you convert Celsius to Kelvin?
-To convert a temperature from Celsius to Kelvin, you add 273 to the Celsius temperature. For example, 25 degrees Celsius is equivalent to 298 Kelvin (25 + 273 = 298 K).
What does it mean for pressure and temperature to be directly proportional?
-When pressure and temperature are directly proportional, it means that if the temperature increases, the pressure also increases, and if the temperature decreases, the pressure decreases as well. This relationship holds true as long as the volume and the amount of gas are constant.
What is the general form of the equation used in Gay-Lussac's Law?
-The general form of the equation used in Gay-Lussac's Law is p1/T1 = p2/T2, where p1 and p2 are the initial and final pressures, and T1 and T2 are the initial and final temperatures in Kelvin.
How do you solve for the final temperature in the given problem with a gas cylinder?
-To solve for the final temperature, you first convert the initial temperature from Celsius to Kelvin, then use the initial pressure and the final pressure in the Gay-Lussac's Law equation to find the final temperature in Kelvin. After that, you convert the final temperature back to Celsius.
What is the expected change in pressure when the temperature of a gas increases, according to the video?
-When the temperature of a gas increases, the pressure of the gas is also expected to increase, as they are directly proportional to each other.
What is the relationship between the initial and final states in the given helium balloon problem?
-The relationship between the initial and final states in the helium balloon problem is given by the Gay-Lussac's Law equation, where the initial pressure and temperature are known, and the final pressure is solved for after the temperature increases.
How do you calculate the final pressure of helium in the balloon when the temperature changes?
-You use the initial pressure and temperature to find the final pressure using the Gay-Lussac's Law equation. After substituting the known values into the equation, you solve for the final pressure (p2) by cross-multiplying and simplifying the equation.
What is the final pressure of the helium in the balloon when the temperature changes from 22 degrees Celsius to 45 degrees Celsius?
-The final pressure of the helium in the balloon is 115 kilopascals, calculated using the Gay-Lussac's Law equation with the given initial and final temperatures converted to Kelvin.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)