CONTOH SOAL : Suhu dan Kalor
Summary
TLDRThe script is a tutorial discussing heat and energy calculations. It explains the conversion of calories to joules, specifically for water, and uses the formula Q=mcΔT to calculate the heat absorbed or released. The tutorial covers examples such as heating water from 10°C to 30°C and determining the final temperature after applying a certain amount of heat energy. It also includes converting calories to joules for calculations and emphasizes unit consistency.
Takeaways
- 🔍 The script discusses a problem related to temperature and heat, starting with a simple but important question about converting calories to joules per kilogram per degree Celsius.
- 📐 The relationship between calories and joules is established, where 1 calorie is approximately equal to 4.18 joules.
- 🔄 The script explains the conversion from calories per gram-degree Celsius to joules per kilogram-degree Celsius, taking into account the mass conversion from grams to kilograms.
- 💧 A practical problem is presented involving heating 3 kg of water from 10°C to 30°C, using the specific heat capacity of water.
- ♨️ The formula for calculating the heat absorbed by the water is provided, which is the product of mass, specific heat capacity, and the change in temperature.
- 🔢 The calculation is demonstrated, showing that the heat absorbed by the water can be determined by multiplying the mass of the water by the specific heat capacity and the temperature change.
- 🌡 Another problem involves heating 100 grams of water from 25°C using 1000 calories of energy, emphasizing the importance of unit consistency.
- 🔄 The final temperature after heating is calculated by adding the temperature change to the initial temperature.
- 💦 A third problem calculates the amount of heat received by 300 grams of water when heated from 30°C to 50°C, using the specific heat capacity of water.
- 🔗 The script highlights the need to convert calories to joules for the final answer, using the conversion factor of 1 calorie to 4.18 joules.
Q & A
What is the relationship between a calorie and a joule?
-One calorie is approximately equal to 4.18 joules.
How can you convert calories per gram-degree Celsius to joules per kilogram-degree Celsius?
-You multiply the calories per gram-degree Celsius by 4.18 to convert to joules per gram-degree Celsius, then divide by 0.001 to convert grams to kilograms.
What is the specific heat capacity of water in joules per kilogram-degree Celsius?
-The specific heat capacity of water is 4200 joules per kilogram-degree Celsius.
How much heat does 3 kg of water at 10°C need to be heated to 30°C?
-The heat required can be calculated using the formula Q = mcΔT, where m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
If 1000 calories of energy are used to heat 100 grams of water at 25°C, what will be the final temperature?
-First, convert the calories to joules (1000 calories * 4.18 joules/calorie), then use the formula Q = mcΔT to find the change in temperature. The final temperature is the initial temperature plus the change in temperature.
How much heat does 300 grams of water need to increase its temperature from 30°C to 50°C?
-The heat required is calculated using the formula Q = mcΔT, where m is the mass of the water, c is the specific heat capacity (1 calorie per gram-degree Celsius), and ΔT is the change in temperature.
What is the final temperature of water after heating if the initial temperature is 25°C and the energy provided is 1000 calories?
-After converting the calories to joules (1000 * 4.18), use the formula Q = mcΔT to find the change in temperature and add it to the initial temperature.
What is the specific heat capacity of a substance and how is it used in calculations?
-The specific heat capacity is the amount of heat required to raise the temperature of one kilogram of a substance by one degree Celsius. It is used in the formula Q = mcΔT to calculate the heat absorbed or released by a substance.
How do you ensure the units are consistent when calculating heat energy in physics problems?
-You must ensure that the units for mass, temperature change, and specific heat capacity are consistent. For example, if the specific heat capacity is given in calories per gram-degree Celsius, you must convert it to joules per kilogram-degree Celsius if the mass is given in kilograms.
What is the significance of the specific heat capacity in the context of the provided script?
-The specific heat capacity is significant as it helps determine the amount of heat needed to change the temperature of a substance, which is a key concept in the script's discussion of heat and temperature.
How do you calculate the final temperature of a substance after heat is added, as described in the script?
-You use the formula Q = mcΔT to find the heat absorbed (Q), then use the specific heat capacity (c) and the mass (m) to find the temperature change (ΔT). The final temperature is the initial temperature plus ΔT.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

The First Law of Thermodynamics: Internal Energy, Heat, and Work
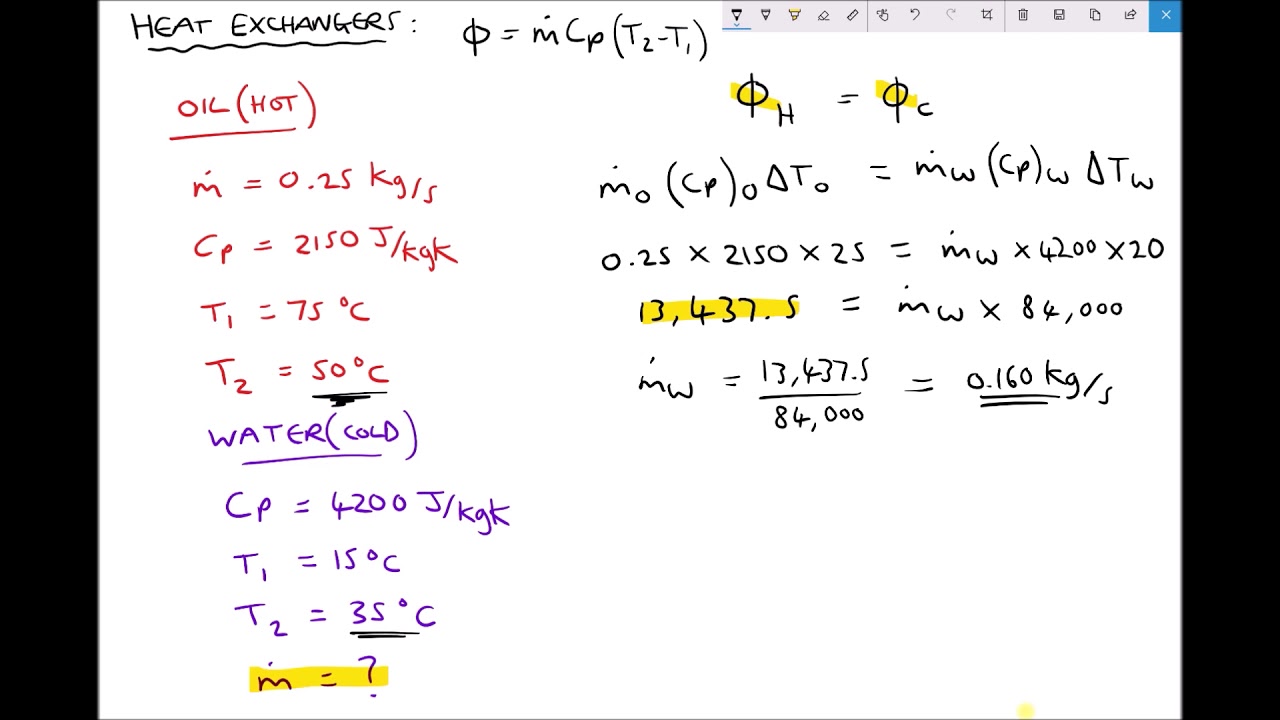
Calculating Rate of Heat Transfer Between Two Working Fluids of a Heat Exchanger

Kalor - Fisika Kelas X
Suhu dan Kalor Fisika Kelas 11 - Part 3 : Kalor dan Azas Black

Calculating the Energy to Melt Ice

Termodinamika • Part 4: Hukum Kedua Termodinamika, Mesin Carnot & Mesin Pendingin
5.0 / 5 (0 votes)
