The Periodic Table EXPLAINED | Chemical Families and Periodic Trends
Summary
TLDRThis video script offers an insightful look into the periodic table's history and organization. It highlights the contributions of Dmitri Mendeleev and Henry Moseley, leading to the current structure based on atomic number. The script explains the periodic law, grouping of elements into families like alkali metals, alkaline earth metals, transition metals, halogens, and noble gases, and discusses key trends such as atomic radius, ionization energy, and electronegativity. It also covers how these trends predict element properties, making the periodic table an essential tool in chemistry.
Takeaways
- 🌐 The periodic table is an iconic symbol in chemistry, recognized worldwide.
- 📚 There have been many ways to organize elements, but the current periodic table is the most widely accepted.
- 🔬 Dmitri Mendeleev's periodic table organized elements by increasing atomic mass and predicted properties of undiscovered elements.
- 🔍 Henry Moseley's research led to the discovery of the atomic number, which rearranged the periodic table.
- 📊 The modern periodic table is organized by increasing atomic number, leading to the periodic law.
- 🔑 The periodic law states that elements with similar properties are grouped together when organized by atomic number.
- 📈 The table is divided into rows (periods) and columns (groups or families), with three broad categories: metals, non-metals, and metalloids.
- 🔬 Metalloids like silicon have properties of both metals and non-metals and are known as semiconductors.
- 📚 Specific groups on the periodic table include alkali metals, alkaline earth metals, transition metals, halogens, and noble gases.
- 📉 Trends in the periodic table include atomic radius, ionization energy, and electronegativity, which can predict element properties.
- 🔋 Ionization energy increases from left to right across a period and decreases as you move down a column.
- 🔑 Electronegativity increases from left to right across a period and decreases as you move down a column, with noble gases having zero electronegativity.
Q & A
Who is credited with designing the first widely accepted periodic table?
-The first widely accepted periodic table was designed by a Russian scientist named Dmitri Mendeleev.
How did Dmitri Mendeleev organize the elements in his periodic table?
-Dmitri Mendeleev organized the elements by increasing atomic mass, which allowed him to predict the properties of other elements and even predict the existence of undiscovered elements.
What significant discovery by Henry Moseley led to the modern periodic table?
-Henry Moseley's research involving X-rays of elements led to the discovery of the atomic number, which is the exact number of protons in the nucleus of an atom. This discovery prompted the rearrangement of the periodic table to organize elements by increasing atomic number.
What is the periodic law and how does it relate to the organization of the periodic table?
-The periodic law states that when elements are organized by increasing atomic number, they will be grouped by common properties. The properties of elements can be predicted by trends and patterns.
What are the three broad categories of elements in the periodic table?
-The three broad categories of elements in the periodic table are metals, non-metals, and metalloids.
How does the property of elements change as you move from left to right across the periodic table?
-As you move from left to right across the periodic table, elements become less metallic. This is due to the increase in the positive charge of the nucleus, which pulls the electron energy levels closer, making atoms smaller and less metallic.
What are metalloids and where are they located on the periodic table?
-Metalloids are elements that have properties of both metals and non-metals. They are located near the stair-step line that separates metals from non-metals on the periodic table.
What are the common families or groups of elements found in the same column of the periodic table?
-Common families or groups of elements found in the same column of the periodic table include alkali metals, alkaline earth metals, transition metals, halogens, and noble gases.
How does atomic radius change as you move down a column in the periodic table?
-As you move down a column in the periodic table, atoms get larger because each time you move down a row, you add another energy level.
What is ionization energy and how does it change across the periodic table?
-Ionization energy is the energy required to remove an electron from the valence shell. It increases from left to right across a period and decreases as you move down a column.
How does electronegativity change as you move across and down the periodic table?
-Electronegativity increases from left to right across the periodic table and decreases as you move down a column. This is because atoms want to achieve a stable electron configuration, typically with eight electrons in their outermost shell.
Which element has the highest electronegativity and why?
-Fluorine has the highest electronegativity because it is the farthest to the right and top of the periodic table, indicating a strong ability to attract electrons.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

A Tour of The Periodic Table

#3 Química 10º ano - Tabela Periódica 🧪

TABEL PERIODIK MODERN | MENENTUKAN GOLONGAN DAN PERIODE
Ang Periodic Table: Brief History in Tagalog

2.1 Atomic Theory and Structure & Introduction to the Periodic Table of the Elements | Chemistry
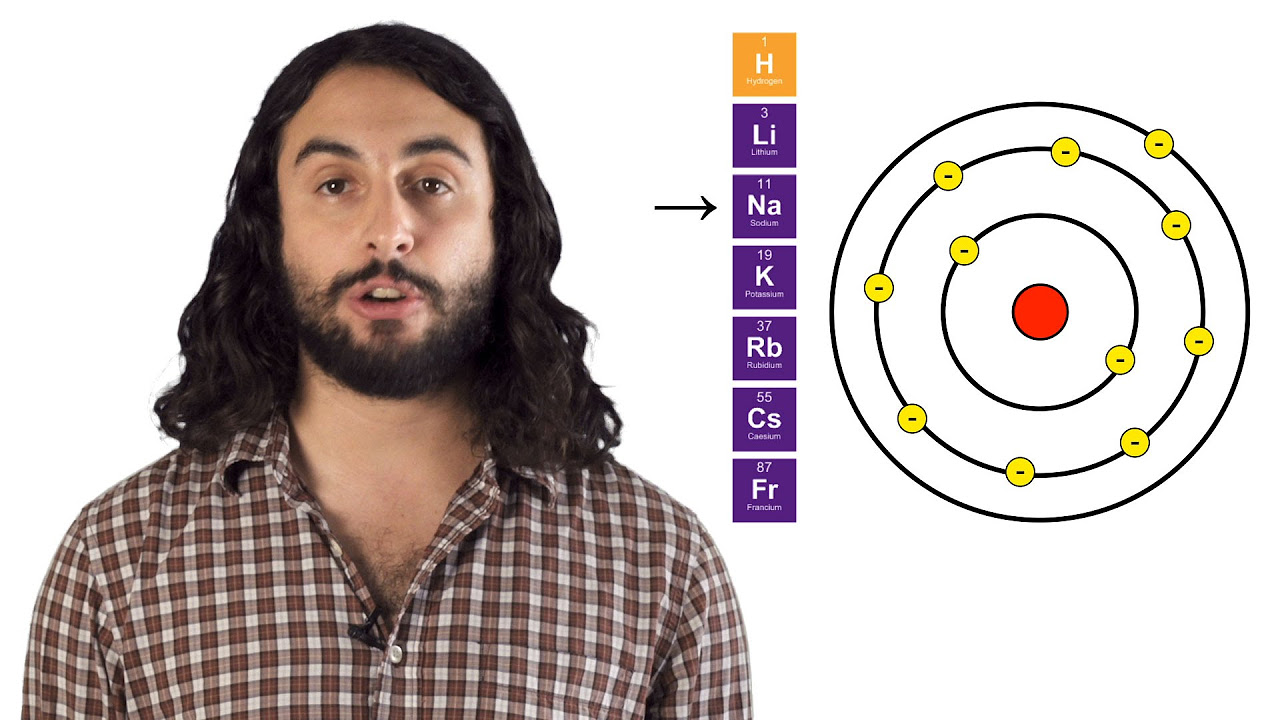
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
5.0 / 5 (0 votes)
