Molecular structure of fructose | Macromolecules | Biology | Khan Academy
Summary
TLDRThis video script delves into the chemistry of fructose, often known as fruit sugar, highlighting its distinction as the sweetest of all monosaccharides. It compares fructose with glucose, both having the same chemical formula but different structures, making them structural isomers. The script explains the key structural difference in their carbonyl group placement and how this affects their cyclical forms, pyranose for glucose and furanose for fructose. It also touches on the formation of sucrose, a disaccharide composed of glucose and fructose, through dehydration synthesis, emphasizing the importance of understanding these sugars in biological and chemical contexts.
Takeaways
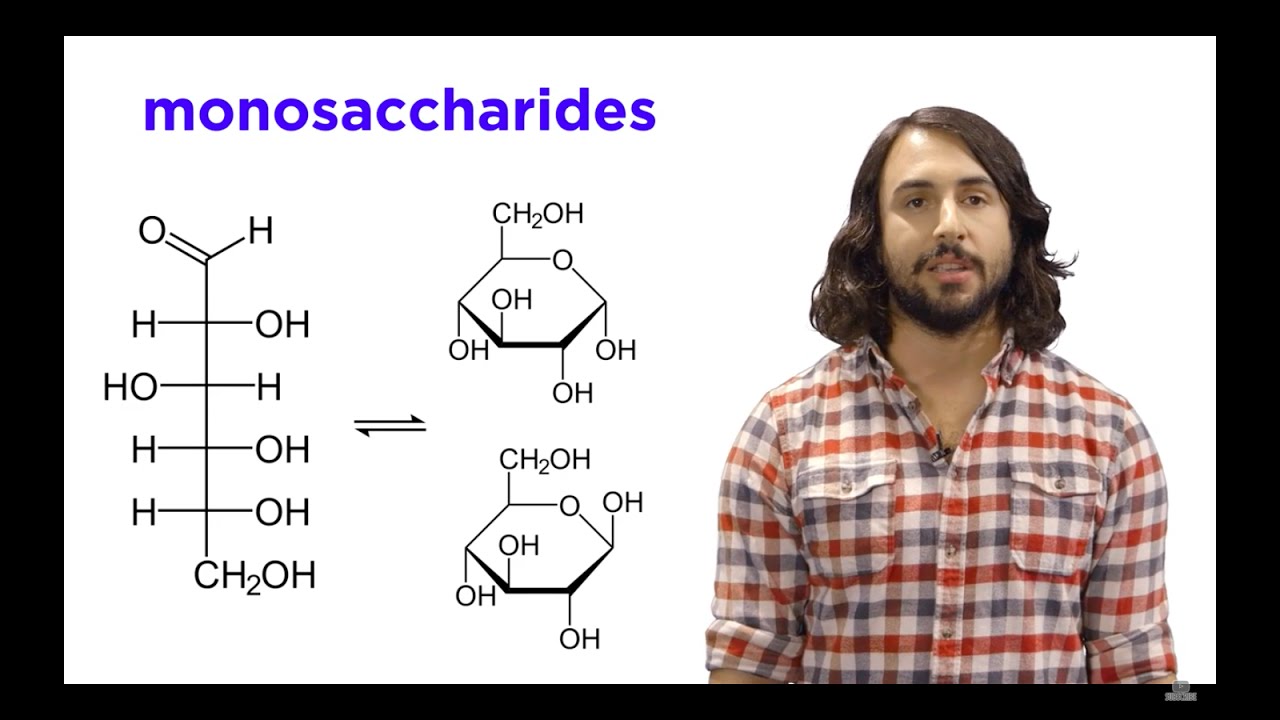
- 🍬 Glucose and fructose are both monosaccharides with the same chemical formula (C6H12O6) but different structures, making them structural isomers.
- 🍇 Fructose is often referred to as fruit sugar and is the sweetest of all sugars when in its monosaccharide form.
- 🔍 The primary structural difference between glucose and fructose is the location of the carbonyl group; glucose has it on the first carbon, while fructose has it on the second.
- 🧪 Glucose is categorized as an aldehyde due to its carbonyl group at the end of the carbon chain, whereas fructose is a ketone with the carbonyl group in the middle.
- 🔄 Both glucose and fructose can exist in both straight chain and cyclic forms, with glucose typically forming a six-member ring (pyranose) and fructose a five-member ring (furanose).
- 🌐 The cyclic forms of glucose and fructose are formed through an intramolecular reaction where the hydroxyl group's oxygen on the fifth carbon attacks the carbonyl group.
- 🍬 Sucrose, or table sugar, is a disaccharide composed of glucose and fructose linked together through a glycosidic bond.
- 🔗 The bond formation in sucrose occurs through a dehydration synthesis reaction, where a water molecule is removed and the glucose and fructose are joined.
- 📚 Drawing and understanding the structures of glucose and fructose is fundamental for students of biology, chemistry, and organic chemistry.
- 📉 The cyclic forms of these sugars are prevalent in nature, with glucose commonly found as a pyranose and fructose typically as a furanose.
- 📚 The script emphasizes the importance of recognizing and distinguishing between the aldehyde and ketone functional groups in sugar molecules.
Q & A
What is fructose, and why is it significant?
-Fructose is a monosaccharide often known as fruit sugar. It is significant because it is the sweetest of all the sugars and plays a crucial role in various biological and chemical processes.
How does the chemical formula of fructose compare to glucose?
-Both fructose and glucose have the same chemical formula, which includes six carbons, 12 hydrogens, and six oxygens. Despite having the same formula, they are different molecules due to the different bonding of their atoms, making them structural isomers.
What is the main structural difference between glucose and fructose?
-The main structural difference between glucose and fructose is the position of the carbonyl group. In glucose, the carbonyl group is attached to the first carbon, while in fructose, it is attached to the second carbon.
What functional groups are present in glucose and fructose due to their carbonyl groups?
-In glucose, the carbonyl group at the end of the carbon chain makes it an aldehyde, while in fructose, the carbonyl group in the middle of the chain makes it a ketone.
What are the cyclical forms of glucose and fructose typically referred to as?
-The cyclical form of glucose is typically referred to as a pyranose, which is a six-member ring with one oxygen. Fructose is typically found in a furanose form, which is a five-member ring with one oxygen.
How does the formation of a cyclical form in fructose differ from that in glucose?
-The formation of a cyclical form in fructose differs from glucose because the carbonyl group in fructose is one carbon further down the chain, resulting in a five-member ring (furanose) instead of a six-member ring (pyranose).
What is the role of the hydroxyl group in the formation of cyclical forms of glucose and fructose?
-The oxygen in the hydroxyl group attached to the fifth carbon in both glucose and fructose forms a bond with the carbon in the carbonyl group, leading to the release of a double bond that can then form a bond with a hydrogen proton, resulting in the cyclical form.
Why is fructose considered the sweetest of all monosaccharides?
-The exact reason why fructose is the sweetest of all monosaccharides is not detailed in the script, but it is a known fact that the molecular structure of fructose allows it to bind more effectively to sweetness receptors on the tongue.
What is sucrose, and how is it related to glucose and fructose?
-Sucrose is a disaccharide composed of glucose and fructose. It is formed through a dehydration synthesis reaction where a glycosidic bond is created between the first carbon of glucose and the second carbon of fructose.
How does the process of dehydration synthesis contribute to the formation of disaccharides like sucrose?
-Dehydration synthesis involves the removal of a water molecule when two monosaccharides join. In the case of sucrose, a water molecule is removed when the hydroxyl group of glucose forms a bond with the carbonyl carbon of fructose, creating a glycosidic linkage.
What is the significance of glycosidic linkages in the formation of disaccharides and polysaccharides?
-Glycosidic linkages are the chemical bonds that connect monosaccharides to form disaccharides and longer chains to form polysaccharides. They are fundamental to the structural integrity and function of these complex carbohydrates.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)