KONSEP DASAR REAKSI REDOKS
Summary
TLDRThis chemistry lesson script delves into the fundamental concept of redox reactions, essential for understanding changes in oxidation states. It explains redox through oxygen-based reactions and electron transfer, using examples to illustrate oxidation as gaining oxygen and reduction as losing it. The script simplifies the concept with mnemonic devices and explores auto-redox reactions, where a single substance acts as both an oxidizer and a reducer. It also touches on the maximum and minimum oxidation states of elements in Group 7A, providing a comprehensive guide to identifying redox reactions.
Takeaways
- 😀 The video is an educational tutorial on the basic concept of redox reactions in chemistry.
- 🔍 It begins by explaining the meaning of 'redox', which is derived from 'reduction' and 'oxidation'.
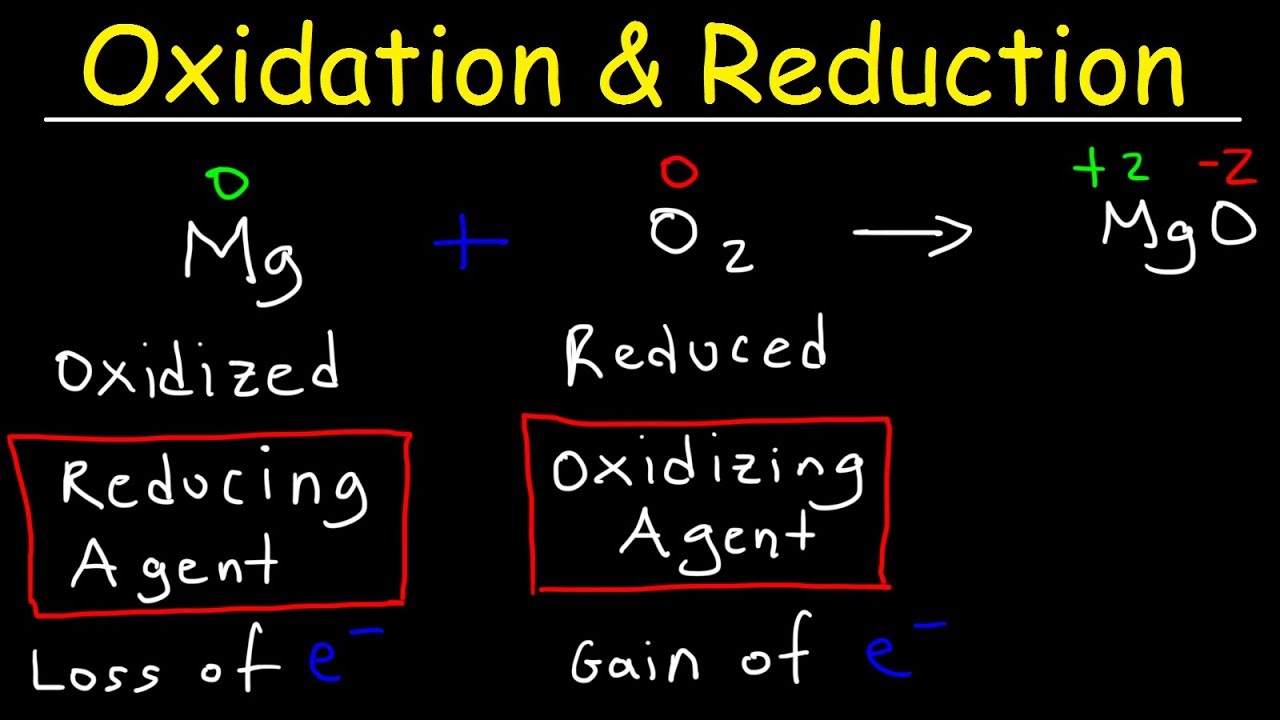
- 🌟 The tutorial discusses the concept of redox reactions based on oxygen, where oxidation involves capturing oxygen and reduction involves releasing it.
- 🔋 It also covers redox reactions based on electron transfer, with oxidation being the loss of electrons and reduction being the gain of electrons.
- 📚 Examples are provided to illustrate oxidation and reduction processes, such as the reaction of carbon with oxygen to form carbon dioxide.
- 🔬 The script introduces a mnemonic device to remember redox reactions involving oxygen and electron transfer.
- 📈 It explains how to determine if a reaction is a redox reaction by checking for changes in oxidation states and the presence of free elements.
- 🛠️ The tutorial provides a trick to identify autoredox reactions, which occur when an element's oxidation state is between its minimum and maximum values.
- 🧩 It gives examples of autoredox reactions, such as the reaction of chlorine gas with sodium hydroxide to form sodium chloride and water.
- 🔑 The script emphasizes the importance of understanding the oxidation states of elements in redox reactions to identify the roles of oxidants and reductants.
- 📝 It concludes by encouraging viewers to like, subscribe, and comment for more educational content and mentions the availability of a summary on Instagram.
Q & A
What is the main topic of the video script?
-The main topic of the video script is the basic concept of redox reactions in chemistry.
What is the meaning of 'redoks' in the context of the script?
-'Redoks' is a term derived from 'reduksi' (reduction) and 'oksidasi' (oxidation), referring to the process involving both reduction and oxidation in a chemical reaction.
What does the script suggest for understanding redox reactions based on oxygen?
-The script suggests understanding redox reactions by looking at the presence or absence of oxygen (O2), where oxidation is the gain of oxygen and reduction is the loss of oxygen.
What is the significance of electrons in redox reactions as mentioned in the script?
-Electrons play a crucial role in redox reactions. Oxidation is when a substance loses electrons, and reduction is when a substance gains electrons.
Can you provide an example of a redox reaction from the script?
-An example from the script is the reaction of magnesium (Mg) with hydrochloric acid (HCl) to form magnesium chloride (MgCl2) and hydrogen gas (H2), where Mg undergoes oxidation and HCl undergoes reduction.
What is the concept of 'auto-redox' reactions mentioned in the script?
-Auto-redox reactions, also known as autoredox or disproportionation reactions, are reactions where a single substance is both oxidized and reduced in the same reaction.
How does the script explain the relationship between the oxidation states of elements in redox reactions?
-The script explains that in redox reactions, the change in oxidation states indicates whether a substance is being oxidized (increasing oxidation state) or reduced (decreasing oxidation state).
What is the role of oxygen in redox reactions according to the script?
-According to the script, oxygen typically acts as an oxidizing agent in redox reactions, meaning it is involved in the process of oxidation by accepting electrons.
How can one determine if a reaction is a redox reaction based on the script?
-The script suggests that to determine if a reaction is a redox reaction, one should look for changes in the oxidation states of the elements involved and the presence of both oxidation and reduction processes.
What is the significance of the oxidation state changes in identifying autoredox reactions in the script?
-The script indicates that for autoredox reactions, the oxidation state of an element should change from its minimum to its maximum, indicating that the same substance is undergoing both oxidation and reduction.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

[MASTER KIMIA SPM] KIMIA KSSM TING 5 : KESEIMBANGAN REDOKS

BALANCEAMENTO POR OXIRREDUÇÃO - PASSO A PASSO

REAKSI REDOKS - SIMPLE KONSEP - KIMIA (Kursus Online Rp8.000 per BULAN : cek deskripsi)
Oxidation and Reduction Reactions - Basic Introduction

Persamaan reaksi redoks

Penyetaraan Reaksi Redoks Metode Bilangan Oksidasi | Kimia SMA | Tetty Afianti
5.0 / 5 (0 votes)