CDM Tutorial | Introduction to Clinical Data Management
Summary
TLDRIn this video, Rupa introduces the concept of Clinical Data Management (CDM), an essential process in healthcare that involves collecting, storing, managing, and validating data from clinical trials. This ensures the accuracy and reliability of data used in medical research and treatment decisions. The video emphasizes the crucial role of clinical data managers in maintaining high-quality data, which is vital for the safety and efficacy of new medical interventions. Viewers will gain insights into the data collection process, data standards, monitoring, privacy, and the tools and career opportunities in this field.
Takeaways
- 😀 Clinical Data Management (CDM) is crucial for collecting, storing, managing, and validating data from clinical trials.
- 😀 CDM ensures the accuracy, reliability, and consistency of data, which is essential for making informed decisions in healthcare.
- 😀 Effective data management directly influences the safety and efficacy of new medical treatments, devices, and strategies.
- 😀 Without proper data management, medical advancements and treatments would not be possible.
- 😀 Clinical data managers play a pivotal role in the management of clinical data, ensuring high data quality for researchers.
- 😀 Key responsibilities of clinical data managers include database design, data monitoring, validation, compliance, and preparing reports.
- 😀 CDM also focuses on ensuring data security, confidentiality, and adherence to regulatory standards.
- 😀 The role of a clinical data manager requires attention to detail, problem-solving skills, and a deep understanding of clinical research.
- 😀 This course will cover various aspects of CDM, including data collection, monitoring, privacy, ethics, and career opportunities in the field.
- 😀 CDM tools, software, and industry standards will be explored to give a deeper understanding of the profession.
- 😀 This video series is designed for medical professionals, students, and anyone interested in the behind-the-scenes work in healthcare advancement.
Q & A
What is clinical data management (CDM)?
-Clinical data management (CDM) involves collecting, storing, managing, and validating data from clinical trials. It ensures that the data is accurate, reliable, and consistent, which is crucial for making decisions about new medical treatments.
Why is clinical data management important?
-CDM is important because without effective data management, we wouldn't have the treatments we rely on today. High-quality data ensures the safety and efficacy of new medical interventions, ultimately saving lives and enabling breakthroughs in disease management.
How does clinical data management impact healthcare advancements?
-CDM plays a vital role in healthcare advancements by ensuring that the data collected during clinical trials is accurate and reliable. This allows for the development of effective medical treatments, such as drugs and devices, which lead to improved patient outcomes.
What is the role of a clinical data manager?
-A clinical data manager is responsible for designing and managing databases for clinical research, ensuring compliance with regulatory bodies, monitoring and validating data, maintaining data security and confidentiality, and preparing data for statistical analysis.
What skills are required for a clinical data manager?
-A clinical data manager must have meticulous attention to detail, strong problem-solving skills, and a profound understanding of clinical research. They are also responsible for ensuring data quality, so researchers can trust the data they are working with.
What will be covered in this course on clinical data management?
-This course will cover topics such as the data collection process, data standards, database design, data monitoring, data privacy, ethics, and much more. It will also discuss the tools, software, and career opportunities in the field of clinical data management.
What is the significance of data privacy and ethics in clinical data management?
-Data privacy and ethics are critical in clinical data management because clinical trials often involve sensitive patient information. It's essential to ensure that this data is protected and that ethical guidelines are followed in the management and use of clinical data.
How do clinical data managers ensure data compliance with regulatory bodies?
-Clinical data managers ensure compliance with regulatory bodies by implementing procedures that meet legal and ethical standards, validating data accuracy, and ensuring that all required documentation is in place for audits and inspections.
What tools and software are commonly used in clinical data management?
-Clinical data managers typically use specialized software tools for data collection, database management, monitoring, and validation. These tools help streamline processes, ensure accuracy, and maintain regulatory compliance in clinical trials.
Who can benefit from understanding clinical data management?
-Anyone interested in healthcare advancements, including medical professionals, students, and individuals considering a career in clinical data management, can benefit from understanding this field. It provides insight into the behind-the-scenes processes that drive clinical trials and medical innovations.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

What is Clinical Data Management? | Clinical Data Management System | Clinical Data Management Jobs
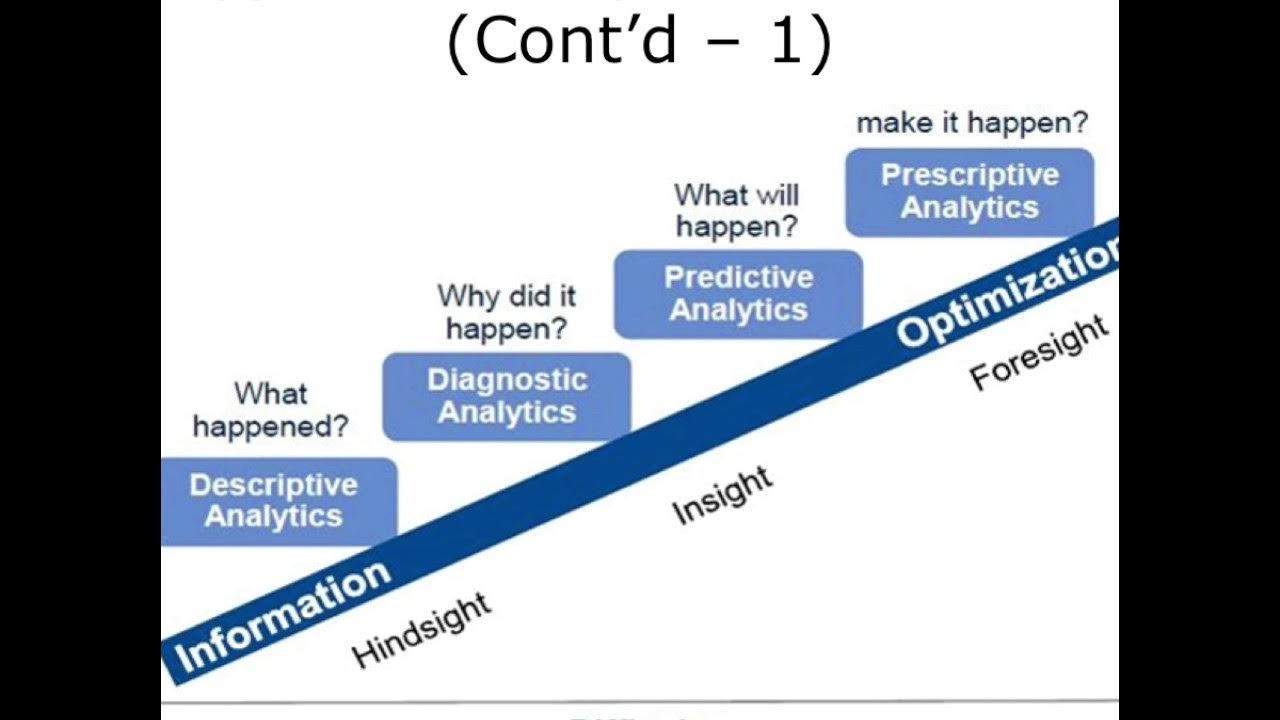
Overview of Health Care Data Analytics

The future of AI in medicine | Conor Judge | TEDxGalway

N-110 Nursing Informatics Lecture 3: Informatics Theory and Practice Applications

4 Types of Healthcare Data Analysts Should Know

Quality Risk Management (QRM) Part 1 of 5
5.0 / 5 (0 votes)