Concentração molal ou molalidade (W) - Brasil Escola
Summary
TLDRThis educational video lesson explains the concept of molal concentration (molality) in chemistry, distinguishing it from molarity. The instructor defines molality as the ratio of moles of solute to kilograms of solvent, highlighting its practical use despite being less commonly applied. The video includes a clear breakdown of the formula, with examples and exercises for practice. Viewers are encouraged to engage with the content by solving problems alongside the teacher, and are reminded about the units involved in molal calculations. The lesson is designed to be interactive, informative, and easy to follow.
Takeaways
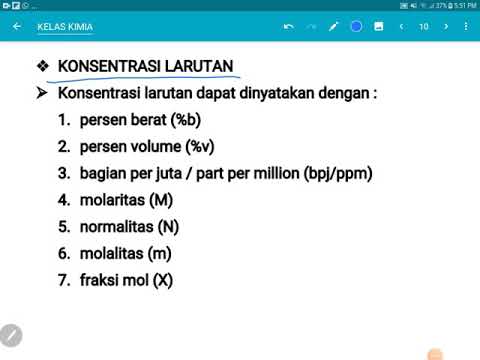
- 😀 The video introduces the concept of molality (concentration molal), which is different from molarity and is less commonly used in chemistry.
- 😀 Molality (w) is defined as the ratio of the number of moles of solute to the mass of the solvent in kilograms, specifically 1 kg of solvent.
- 😀 The formula for calculating molality is: w = n1 / m2, where n1 is the number of moles of solute, and m2 is the mass of the solvent in kilograms.
- 😀 The video encourages understanding molality through practical application, such as solving an exercise, to make the concept clearer.
- 😀 The units of molality are expressed in moles per kilogram (mol/kg).
- 😀 The molality calculation can be simplified by using the formula for moles, where n = mass of solute / molar mass of solute.
- 😀 A specific exercise from the video asks about the molality of a solution containing 1.5 mol of solute (lino3) per 1 kg of solvent (ethanol).
- 😀 It is important to remember that molality refers to the number of moles per kilogram of solvent, not per liter of solution, which is a common point of confusion.
- 😀 The second example involves calculating the molality of a solution with 34.2 g of sucrose dissolved in 200 g of water, resulting in a molality of 0.5 mol/kg.
- 😀 The video emphasizes the importance of converting units when calculating molality, such as converting grams of solvent to kilograms.
- 😀 The video ends by encouraging viewers to review the material, try solving exercises on their own, and engage with the channel for more educational content.
Q & A
What is the molal concentration (molality)?
-Molality is the concentration of a solution, defined as the number of moles of solute per kilogram of solvent.
How is molality different from molarity?
-Molality is based on the mass of the solvent (measured in kilograms), whereas molarity is based on the volume of the solution (measured in liters).
What symbol represents molal concentration?
-Molal concentration is represented by the letter 'w'.
What is the formula for calculating molality?
-The formula for molality is W = N1 / M2, where N1 is the number of moles of solute, and M2 is the mass of the solvent in kilograms.
How can you calculate the number of moles (N1) of solute in a molality calculation?
-The number of moles of solute (N1) can be calculated using the formula N1 = mass of solute / molar mass of solute.
What are the typical units used in molality calculations?
-The units for molality are moles per kilogram (mol/kg), the molar mass is in grams per mole (g/mol), and the mass of the solvent is in kilograms (kg).
What does '1.50 molal of Ln3 in ethanol' mean?
-It means that for every 1 kilogram of ethanol solvent, there are 1.50 moles of Ln3 solute present.
In the problem involving 34.2g of sucrose (C12H22O11) and 200g of H2O, how do you convert the mass of water to kilograms?
-To convert 200g of water to kilograms, divide by 1000, so 200g = 0.2 kg.
What is the molar mass of sucrose (C12H22O11)?
-The molar mass of sucrose is calculated as 342 g/mol, based on the atomic masses of carbon, hydrogen, and oxygen in the molecule.
How do you calculate the molality of a solution given the mass of solute and solvent?
-The molality can be calculated using the formula W = (mass of solute / molar mass of solute) / (mass of solvent in kg). For the sucrose example, the molality was found to be 0.5 mol/kg.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)