KONSENTRASI LARUTAN : MOLARITAS, MOLALITAS, DAN FRAKSI MOL
Summary
TLDRThe video discusses the concept of solution concentration in chemistry, focusing on molarity, molality, and mole fraction. It explains how molarity is the number of moles of solute per liter of solution, while molality measures moles of solute per kilogram of solvent. The video provides several practical examples and calculations to illustrate these concepts, including specific cases involving common compounds like MgSO4 and NaCl. Additionally, it introduces mole fraction as a measure of a component's ratio in a solution, emphasizing the importance of understanding these calculations in chemical practices.
Takeaways
- 🔍 Chemical reactions in the lab and daily life usually occur in solutions, which are homogeneous mixtures of solute and solvent.
- ⚖️ The composition of a solution depends on the ratio of solute to solvent, known as concentration.
- 📏 Molarity (M) is defined as the number of moles of solute per liter of solution, with the formula: Molarity = moles of solute / volume of solution (L).
- 📐 Molarity can also be calculated using mass and molecular weight: Molarity = (mass of solute / molecular weight) × 1000 / volume (mL).
- 💧 Example 1: The molarity of 0.05 moles of MgSO4 in 500 mL of solution is calculated as 0.1 M.
- 🧪 Example 2: The molarity of a solution made by dissolving 5.85 grams of NaCl in 500 mL of water is 0.2 M.
- ⚗️ Molality (m) represents the number of moles of solute per kilogram of solvent, with the formula: Molality = moles of solute / mass of solvent (kg).
- 🧮 Example 3: The molality of 0.2 moles of BaCl2 dissolved in 500 grams of water is calculated to be 0.4 mol/kg.
- 🔢 Mole fraction (X) is the ratio of moles of a specific component to the total moles in a solution.
- 📊 Example 4: The mole fraction of ethanol and water in a mixture of 46 grams of ethanol and 44 grams of water can be calculated to be 0.29 for ethanol and 0.71 for water.
Q & A
What is a solution in the context of chemistry?
-A solution is a homogeneous mixture of a solute and a solvent with a specific composition.
What determines the composition of a solution?
-The composition of a solution depends on the ratio of the amount of solute to the solvent, known as concentration.
What are some common units used to express concentration?
-Common units for expressing concentration include molarity, molality, and mole fraction.
How is molarity defined mathematically?
-Molarity (M) is defined as the number of moles of solute divided by the volume of the solution in liters.
How can molarity be calculated using mass?
-Molarity can also be calculated using the formula: Molarity = (mass of solute in grams / molar mass of solute in g/mol) * (1000 / volume of solution in mL).
What is molality and how is it different from molarity?
-Molality (m) is defined as the number of moles of solute per kilogram of solvent, differing from molarity, which is based on the volume of the solution.
What is the formula for calculating molality?
-Molality can be calculated using the formula: Molality = (moles of solute / mass of solvent in kg).
How is mole fraction calculated?
-Mole fraction is calculated as the number of moles of a particular component divided by the total number of moles of all components in the mixture.
In the example provided, how is the molarity of MgSO4 determined?
-The molarity of MgSO4 is calculated by converting 500 mL to liters (0.5 L) and then applying the formula: Molarity = 0.05 mol / 0.5 L, resulting in 0.1 M.
What is the significance of understanding these concentration concepts in chemistry?
-Understanding concentration concepts is essential for preparing solutions, conducting experiments, and analyzing chemical reactions accurately.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
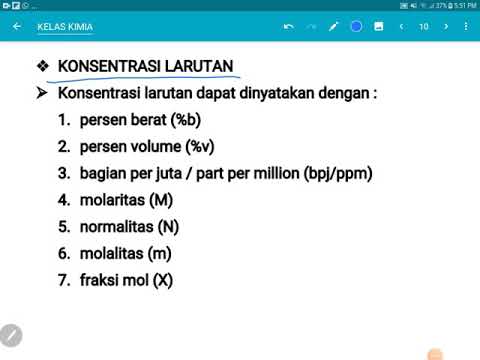
Kelas Kimia : Konsentrasi Larutan (% berat, % volume, ppm / bpj)

SIFAT KOLIGATIF 1
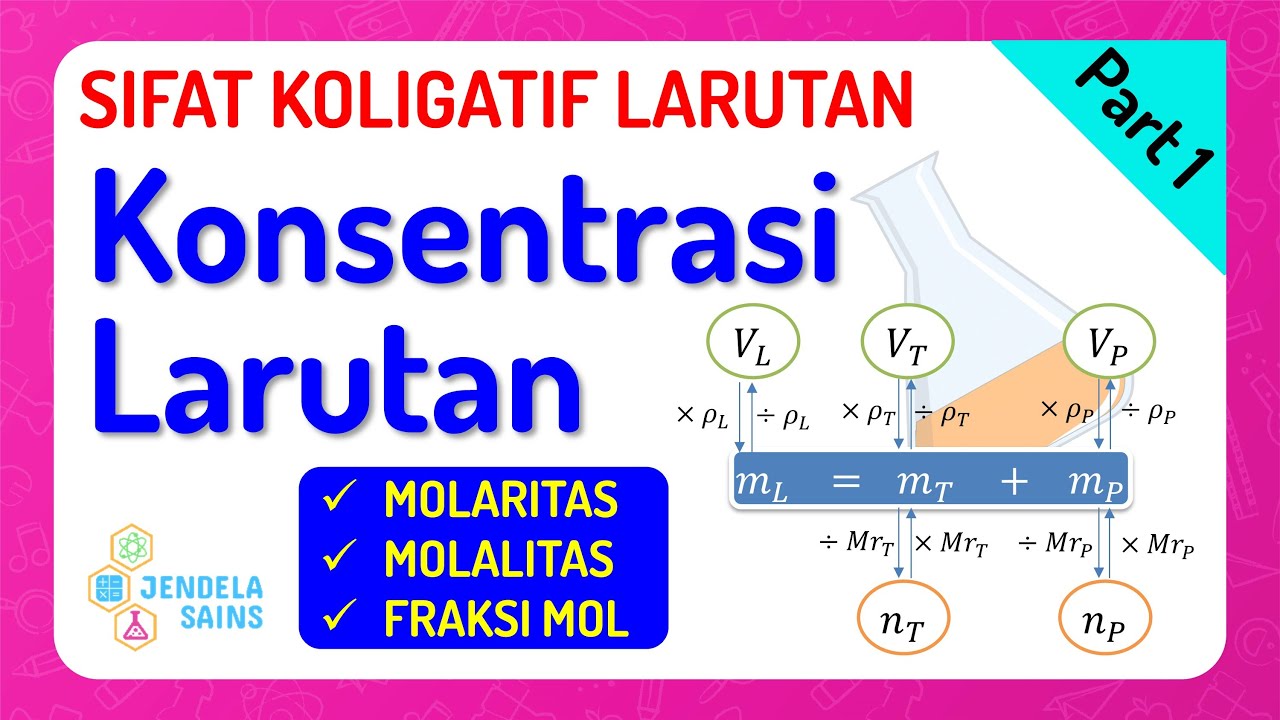
Sifat Koligatif Larutan • Part 1: Konsentrasi (Molaritas, Molalitas, Fraksi Mol)

ruangbelajar - Kimia XII SMA - Pendahuluan Sifat Koligatif

Unit 8 Solutions Concept 2 Notes

An Easy Way to Understanding the Mole and Avogadros Number Part 14
5.0 / 5 (0 votes)