Nitrogen & Phosphorus Cycles: Always Recycle! Part 2 - Crash Course Ecology #9
Summary
TLDRThis episode of Crash Course Ecology delves into the nitrogen and phosphorus cycles, essential for life but often inaccessible in their natural forms. The script explains how nitrogen-fixing bacteria and plants play crucial roles in making these elements available, while human intervention through synthetic fertilizers has dramatically increased their availability, with both benefits for agriculture and potential environmental consequences.
Takeaways
- 🌊 The nitrogen and phosphorus cycles are essential for life, yet these elements are often unavailable in biologically useful forms despite their abundance.
- 💧 Water is abundant in oceans but not directly consumable for hydration, illustrating the concept of elements being present but inaccessible.
- 🌱 Plants are crucial in making nitrogen and phosphorus available to other organisms by assimilating these nutrients from the environment.
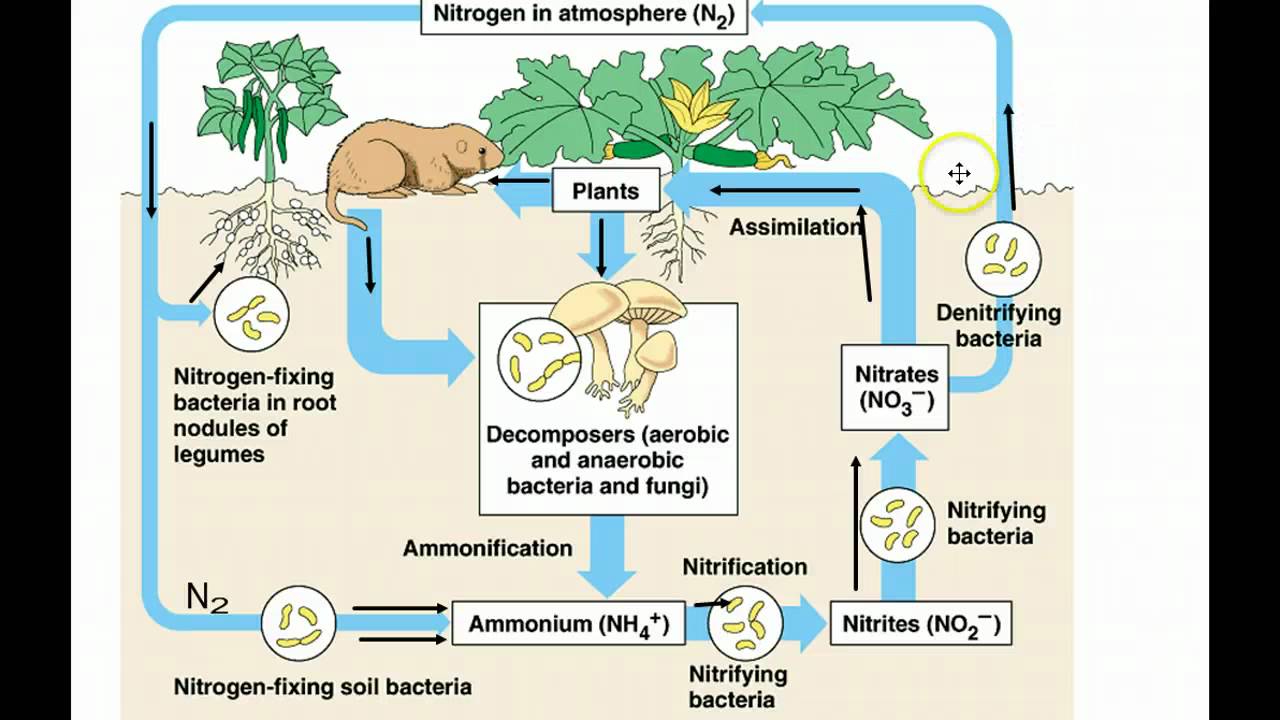
- ⚗️ Nitrogen gas, which makes up 78% of the atmosphere, is difficult for organisms to use due to the strong triple bond between nitrogen atoms.
- 🌿 Nitrogen-fixing bacteria and certain plants, particularly legumes, form symbiotic relationships that allow the conversion of atmospheric nitrogen into a usable form.
- 🔬 Nitrogenase is a unique enzyme that can break the triple bond in nitrogen gas, performed by nitrogen-fixing bacteria.
- 🌩 Lightning and human innovation have also been identified as ways to fix atmospheric nitrogen, contributing to the nitrogen cycle.
- 🔄 The phosphorus cycle does not involve the atmosphere and is primarily driven by the lithosphere, with rocks being a significant source of phosphorus.
- 🪨 Phosphates from eroded rocks are absorbed by plants and enter the food chain, highlighting the importance of the rock-water-plant cycle.
- 🌊 Once in aquatic ecosystems, phosphorus can be trapped in deep bodies of water for extended periods, affecting the phosphorus cycle.
- 🚜 Human use of synthetic fertilizers has dramatically increased the availability of nitrogen and phosphorus, but also led to environmental challenges.
Q & A
Why can't organisms directly utilize the nitrogen and phosphorus that are abundant in the environment?
-Organisms can't directly utilize these elements because they are often in a form that is not biologically available. Nitrogen is mostly found as nitrogen gas (N2) with a strong triple bond that is difficult to break, and phosphorus is often locked in rocks or sediments.
What role do nitrogen-fixing bacteria play in the nitrogen cycle?
-Nitrogen-fixing bacteria convert atmospheric nitrogen (N2) into ammonia (NH3), which then becomes ammonium (NH4+) when mixed with water. This process makes nitrogen biologically available for plants to assimilate and use.
How do plants obtain nitrogen if they can't break the triple bond in nitrogen gas?
-Plants rely on the nitrogen-fixing bacteria that convert atmospheric nitrogen into forms like ammonia or ammonium, which plants can then assimilate.
What is the enzyme that allows nitrogen-fixing bacteria to break the triple bond in nitrogen gas?
-The enzyme is called nitrogenase, which is the only biological enzyme capable of breaking the strong triple bond in nitrogen gas.
What is the significance of the phosphorus cycle in relation to the nitrogen cycle?
-While the nitrogen cycle involves the conversion of atmospheric nitrogen into biologically available forms, the phosphorus cycle deals with the availability of phosphorus from the lithosphere. Both cycles are essential for the production of essential biomolecules like DNA, RNA, and ATP.
Why is phosphorus different from nitrogen in terms of its cycle?
-Phosphorus does not involve the atmosphere and is primarily cycled through the lithosphere. It is found in rocks, particularly in sedimentary rocks that originated from organic matter accumulation in ocean floors and lake beds.
How do plants assimilate phosphorus from the environment?
-Plants assimilate phosphorus when it is dissolved into water from eroded rocks. This dissolved phosphate is immediately available for plant uptake.
What happens to the nitrogen and phosphorus once they are assimilated by animals?
-Animals use the nitrogen and phosphorus to produce amino acids and other biomolecules. When animals excrete waste or die, decomposers break down the organic matter, releasing the nitrogen and phosphorus back into the environment to continue the cycle.
How do humans impact the nitrogen and phosphorus cycles through the use of synthetic fertilizers?
-Humans have significantly increased the availability of nitrogen and phosphorus in ecosystems by introducing synthetic fertilizers, which can lead to environmental issues such as eutrophication and other imbalances.
What is the final destination of phosphorus in the phosphorus cycle?
-The final destination of phosphorus is when it becomes part of deep sedimentary rocks, where it can remain trapped for millions of years before being uplifted and weathered back into the cycle.
Why is it important for organisms to have access to biologically available nitrogen and phosphorus?
-Access to biologically available nitrogen and phosphorus is crucial for the synthesis of essential biomolecules like amino acids, DNA, RNA, and ATP, which are the building blocks of life.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Daur Biogeokimia

KELAS 10 : MATERI EKOSISTEM BAG. 2 (Daur Biogeokimia)

Daur Biogeokimia : daur karbon, daur nitrogen, daur sulfur, daur oksigen, daur fosfor | biologi sma

How the Earth Recycles Elements: Biogeochemical Cycles
Ecosystems Part 2 - Nutrient Cycling

Community Ecology: Feel the Love - Crash Course Ecology #4
5.0 / 5 (0 votes)