Elusidasi Instrumen FT-IR | Membaca Spektra IR
Summary
TLDRIn this video, the presenter explains how to read IR spectra in organic chemistry. The focus is on identifying functional groups, particularly the carbonyl (C=O) group and its various derivatives, such as esters, amides, and aldehydes. The video provides detailed examples, including heptane, cyclohexanol, and butanoic acid, teaching viewers how to recognize different vibrations and spectral patterns. Key concepts like the wave number ranges for specific functional groups, and their respective characteristic peaks, are highlighted. The video aims to enhance the understanding of IR spectroscopy through practical examples and encourages practice for better identification skills.
Takeaways
- 😀 Spectra IR is a technique used to identify functional groups in organic compounds, starting with the presence of a carbonyl group (C=O).
- 😀 The presence of carbonyl groups in a molecule can indicate different functional groups such as anhydride, acid, amide, ester, or aldehyde, depending on their accompanying groups.
- 😀 When no carbonyl group is present, functional groups are identified based on a hierarchy, starting with hydroxyl groups (OH) and halides (CX).
- 😀 Understanding wave numbers is key to reading IR spectra, as different functional groups absorb infrared radiation at specific wave number ranges.
- 😀 Common functional groups, like OH and C=O, have characteristic IR absorption ranges that can be memorized for easier identification.
- 😀 The IR spectrum of hexane shows key CH stretches around 2900–2800 cm-1, helping to identify the presence of CH2 and CH3 groups.
- 😀 Cyclohexanol’s IR spectrum reveals a broad and more spread out O-H stretch, indicating hydrogen bonding, while free hydroxyl groups have a sharper peak.
- 😀 The IR spectrum of cyclohexanol also shows distinct peaks for C-O alcohol groups in the 1000–1100 cm-1 range, and CH2 bending vibrations around 1400 cm-1.
- 😀 In the case of butanoic acid, the key IR features include a sharp C=O stretch around 1700 cm-1 and an O-H stretch around 3300 cm-1, indicating the presence of carboxyl groups.
- 😀 The learning process of reading IR spectra requires frequent practice and familiarity with common functional group patterns to confidently identify compounds.
Q & A
What is the primary focus of this video?
-The primary focus of the video is to teach how to read Infrared (IR) spectra and identify functional groups in organic compounds based on their spectral data.
How can you identify a carbonyl group (C=O) in a compound?
-A carbonyl group (C=O) can be identified in a compound by observing its characteristic IR absorption, which appears in the range of 1700 cm^-1. If it is followed by specific functional groups, like two carbonyl groups, it may indicate an anhydride functional group.
What does the presence of a hydroxyl group (OH) indicate in IR spectra?
-The presence of a hydroxyl group (OH) in IR spectra indicates the presence of alcohols or acids. If the OH group is free, the absorption appears as a sharp peak, while if it is hydrogen-bonded (as in acids), the absorption is broader.
What range of wavenumbers would you find the CH stretching vibrations in the IR spectra?
-CH stretching vibrations typically appear in the IR spectra between 2800 cm^-1 and 3000 cm^-1, which corresponds to the vibrations of CH3 and CH2 groups.
How can you differentiate between CH2 and CH3 bending vibrations in IR spectra?
-CH2 and CH3 bending vibrations are observed in the range of 1300 cm^-1 to 1400 cm^-1. The vibrations can be further differentiated by their specific intensities and symmetry in the spectra.
What does the spectrum of hexane show regarding its functional groups?
-The IR spectrum of hexane shows the CH stretching vibrations in the range of 2800 cm^-1 to 3000 cm^-1, as well as bending vibrations around 1300 cm^-1 to 1400 cm^-1, corresponding to CH2 and CH3 groups.
How does the IR spectrum of cyclohexanol differ from hexane?
-Cyclohexanol’s IR spectrum shows a broad and wide OH stretching band around 3300 cm^-1, indicating a hydrogen-bonded hydroxyl group, while hexane does not have an OH group and only shows CH stretching and bending.
What is the significance of the sharp peak around 1700 cm^-1 in an IR spectrum?
-A sharp peak around 1700 cm^-1 in an IR spectrum typically indicates the presence of a carbonyl group (C=O), which is characteristic of compounds like aldehydes, ketones, acids, and esters.
How can you identify the functional group of an ester in IR spectra?
-An ester can be identified in IR spectra by the C=O stretching absorption around 1735 cm^-1 and the C-O stretching absorption around 1200 cm^-1 to 1300 cm^-1.
What practical step should be taken to improve the understanding of IR spectra?
-To improve understanding of IR spectra, it is essential to practice by analyzing multiple spectra and matching them with known functional groups, reinforcing knowledge of characteristic absorption ranges.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
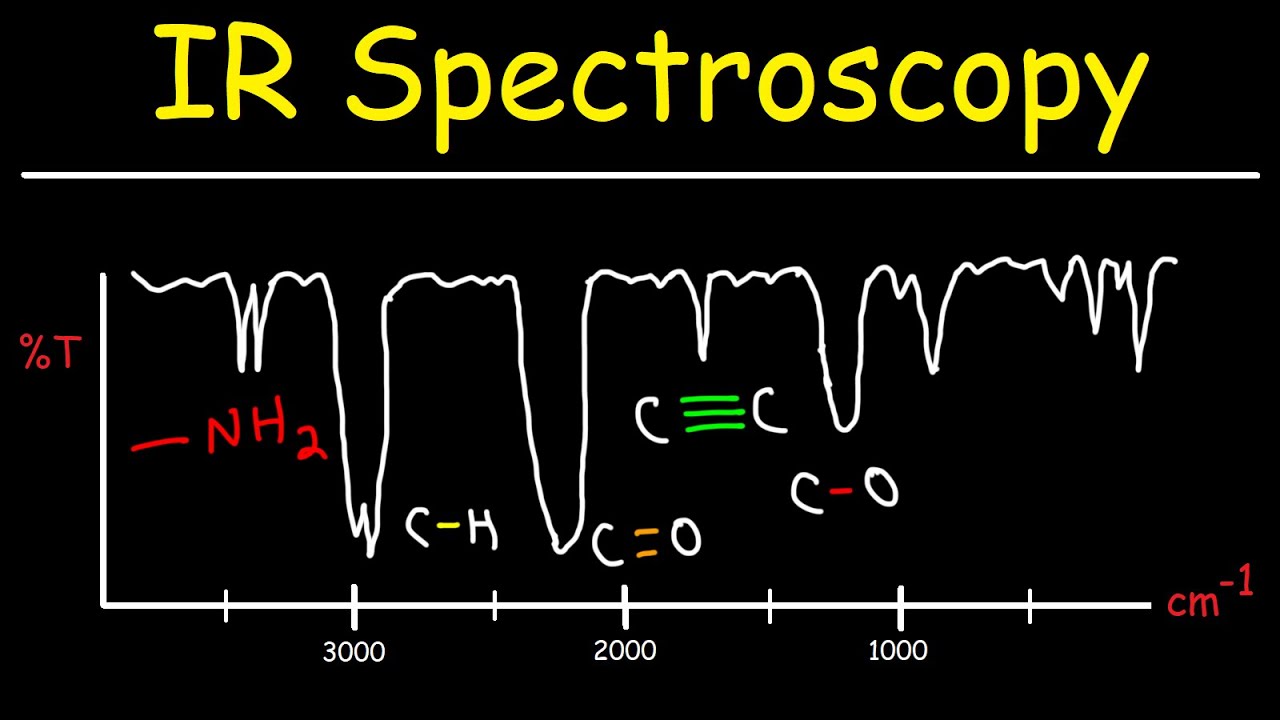
IR Spectroscopy - Basic Introduction
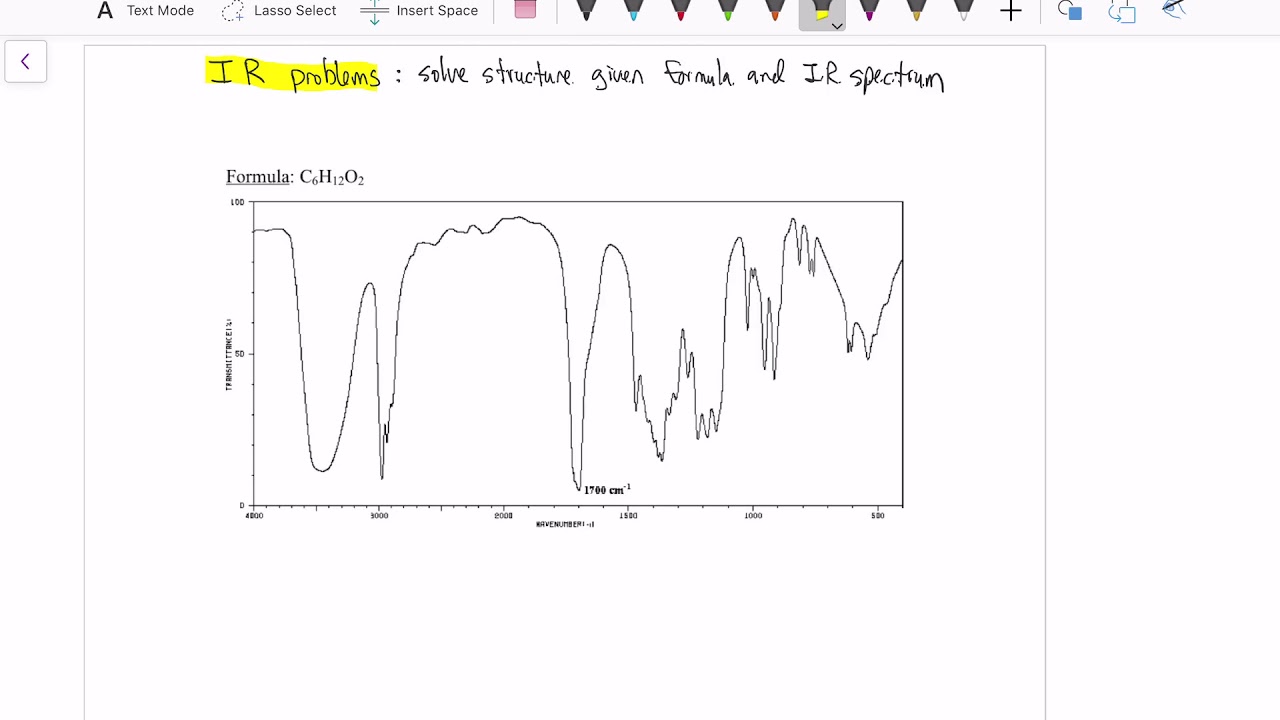
Determine structures from IR spectra

Interpretation of IR Spectra

Cara mudah baca spektra IR | Bahas soal spektra IR | Kupas tuntas 5 soal spektra IR
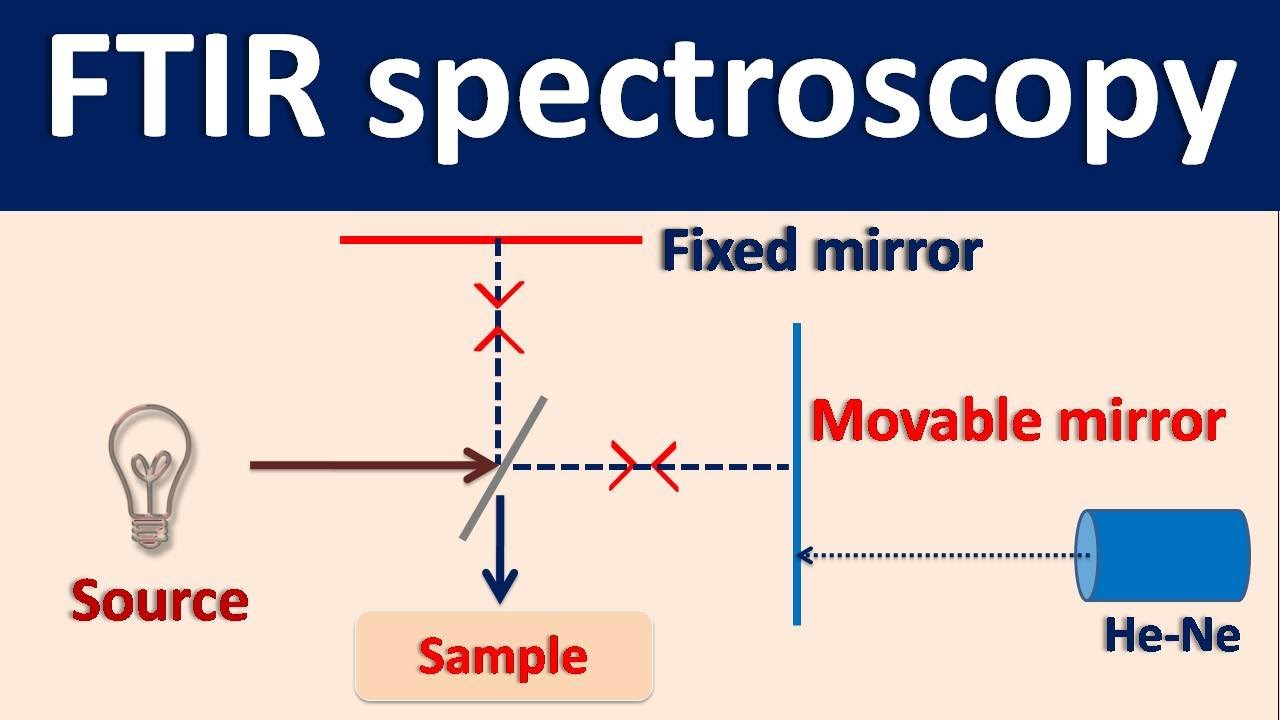
Fourier Transform IR spectroscopy (FTIR) - How it works?

IR spectra for hydrocarbons | Spectroscopy | Organic chemistry | Khan Academy
5.0 / 5 (0 votes)